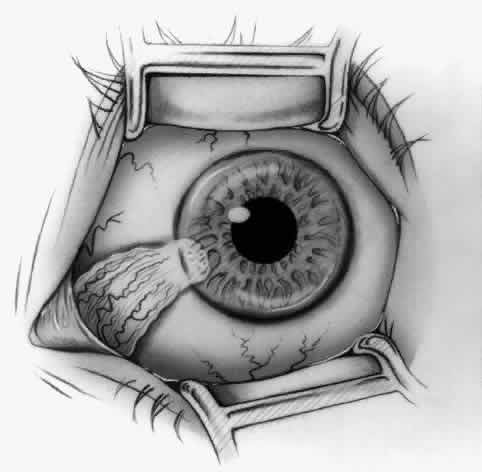
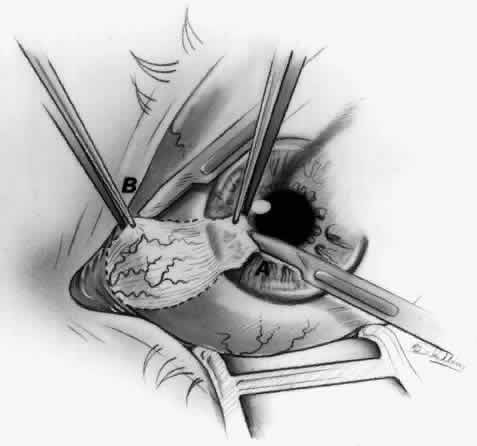
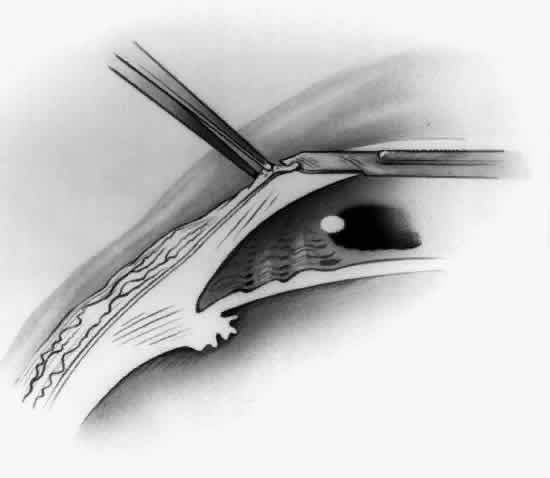
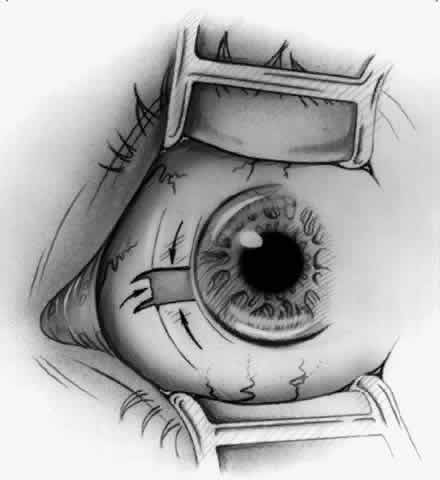
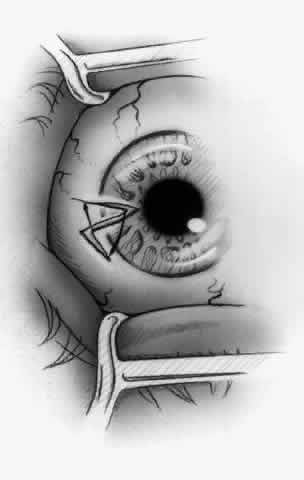
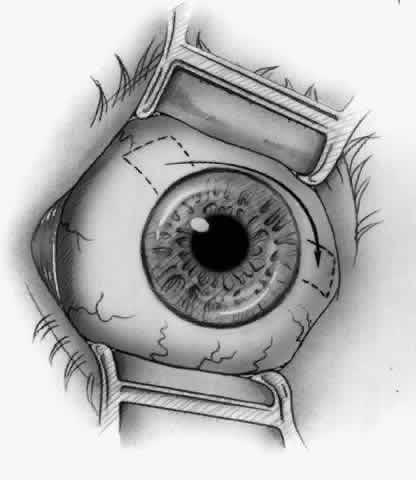
1. Duke-Elder S: System of Ophthalmology, Vol 8, Diseases of the Outer Eye, p 573. St. Louis, CV
Mosby, 1965 2. Arffa RC: Degenerations. In Arffa RC (ed): Grayson's Diseases of the
Cornea, 3rd ed, p 342. St. Louis, CV Mosby, 1991 3. Jaros PA, DeLuise VP: Pingueculae and pterygia. Surv Ophthalmol 33:41, 1988 4. Townsend WM: Pterygium. In Kaufman HE, McDonald MB, Barron BA et al (eds): The
Cornea, p 461. New York, Churchill Livingstone, 1988 5. Karai I, Horiguchi S: Pterygium in welders. Br J Ophthalmol 68:347, 1984 6. Norm M, Franck C: Long-term changes in the outer part of the eye in welders. Acta Ophthalmol 69:382, 1991 7. Adamis AP, Starck T, Kenyon KR: The management of pterygium. Ophthalmol Clin North Am 3(4):611, 1990 8. Awan KJ: The clinical significance of a single unilateral temporal pterygium. Can J Ophthalmol 10:222, 1975 9. Wong WW: A hypothesis on the pathogenesis of pterygiums. Ann Ophthalmol 10:303, 1978 10. Char D: Corneal tumors. In Smolin G, Thoft RA (eds): The Cornea, p 499. Boston, Little, Brown & Co, 1987 11. Lin S, Reiter K, Dreher AW et al: The effect of pterygia on contrast sensitivity and glare disability. Am J Ophthalmol 107:407, 1989 12. Lewallen S: A randomized trial of conjunctival autografting for pterygium in the tropics. Ophthalmology 96:1612, 1989 13. Goldberg L, David R: Pterygium and it relationship to the dry eye in the Bantu. Br J Ophthalmol 60:720, 1976 14. Dake Y, Mukae R, Soda Y et al: Immunohistochemical localization of collagen types I, II, III, and IV in
pterygium tissues. Acta Histochem (Jena) 87(1):71, 1989 15. Mackenzie FD, Hirst LW, Battistutta D, Green A: Risk analysis in the development of pterygia. Ophthalmology 99:1056, 1992 16. Hilgers JHC: Pterygium: Its incidence, heredity and etiology. Am J Ophthalmol 50:635, 1960 17. Forsius H, Eriksson A: Pterygium and its relation to arcus senilis, pinguecula and other similar
conditions. Acta Ophthalmol 40:402, 1962 18. Taylor HR, West SK, Rosenthal FS et al: Corneal changes associated with chronic UV irradiation. Arch Ophthalmol 107:1481, 1989 19. Detels R, Dhir SP: Pterygium: A geographical study. Arch Ophthalmol 78:485, 1967 20. Paton D: Pterygium management based upon a theory of pathogenesis. Trans Am Acad Ophthalmol Otolaryngol 79:603, 1975 21. Forsius H, Eriksson A: Pterygium in an isolated population. Acta Genet Med Gemellol (Roma) 11:397, 1962 22. Austin P, Jakobiec FA, Iwamoto T: Elastodysplasia and elastodystrophy as the pathologic bases of ocular pterygia
and pinguecula. Ophthalmology 90:96, 1983 23. Spencer WH, Zimmerman LE: Conjunctiva. In Spencer WH (ed): Ophthalmic Pathology, vol 1, p 174. Philadelphia, WB Saunders, 1985 24. Cogan DG, Kuwabara T, Howard J: The nonelastic nature of pingueculas. Arch Ophthalmol 61:388, 1959 25. Daroczy J, Vajda K, Kiraly K: Study of the ultrastructure of normal and
pathologic human dermal elastic fibre. In Robert L (ed): Frontiers of
Matrix Biology, Vol 4, Studies on the Biology and Pathology of Skin, p 122. Basel, Karger, 1977 26. Hogan MJ, Alvarado J: Pterygium and pinguecula: Electron microscopic study. Arch Ophthalmol 78:174, 1967 27. Cameron ME: The treatment of beta irradiation necrosis of the sclera. Aust J Ophthalmol 6:86, 1978 28. Goldman KN, Kaufman HE: Atypical pterygium. Arch Ophthalmol 96:1027, 1978 29. Cameron ME: Pterygium Throughout the World, p 141. Springfield, IL, Charles
C Thomas, 1965 30. Kawano K, Uehara F, Ohba N: Lectin-cytochemical study on epithelial mucus glycoprotein of conjunctiva
and pterygium. Exp Eye Res 47:43, 1988 31. Kaneko M, Takaku I, Katsura N: Glycosaminoglycans in pterygium tissues and normal conjunctiva. Jpn J Ophthalmol 30:165, 1986 32. Kaneko M: Proteoglycans from pterygium tissues. Ophthalmic Res 19:170, 1987 33. Pinkerton OD, Hokama Y, Shigemura LA: Immunologic basis for the pathogenesis of pterygium. Am J Ophthalmol 98:225, 1984 34. Rosenthal JW: Chronology of pterygium therapy. Am J Ophthalmol 36:1601, 1953 35. D'Ombrain A: The surgical treatment of pterygium. Br J Ophthalmol 32:65, 1948 36. Rich AM, Keitzman B, Payne T et al: A simplified way to remove pterygia. Ann Ophthalmol 6:739, 1974 37. King JH: The pterygium. Arch Ophthalmol 44:854, 1950 38. Herbstein AU, Donovan JK: Pterygium removal. Br J Ophthalmol 52:162, 1968 39. Prince AE: An accidental divulsion of a pterygium, leading to an improvement in the
regular operation. Arch Ophthalmol 14:16, 1885 40. Tower P: Corrugated silver wire for severing pterygium from the cornea. Am J Ophthalmol 33:1439, 1950 41. Zolli CL: Experience with the avulsion technique in pterygium surgery. Ann Ophthalmol 11:1569, 1979 42. Gibson JBC: Brisbane surgery of pterygium. Trans Ophthalmol Soc Aust 16:125, 1956 43. Hersh PS: Superficial keratectomy. In Hersh PS (ed): Ophthalmic Surgical
Procedures, p 221. Boston, Little, Brown & Co, 1988 44. Coroneo MT: Beheading the pterygium. Ophthalmic Surg 23:691, 1992 45. Alger LJ: Etiology of pterygium recurrence. Am J Ophthalmol 57:450, 1964 46. Youngson RM: Recurrence of pterygium after excision. Br J Ophthalmol 56:120, 1972 47. Sugar HS: A surgical treatment for pterygium based on new concepts as to its nature. Am J Ophthalmol 32:912, 1949 48. Terson A: Structure and pathogenesis of pterygium: With a modified operation. Arch Ophthalmol (Paris) 31:161, 1911 49. Campodonico E: A new procedure in the excision method of pterygium operation. In
Zentmayer W (ed): Transactions of XII International Congress
of Ophthalmology, p 201. Philadelphia, WF Fell, 1922 50. Bangerter A: Pterygium operation and covering of conjunctival defects. Ophthalmologica 106:316, 1943 (in German) 51. Tomas T: Sliding flap of conjunctival limbus to prevent recurrence of pterygium. Refract Corneal Surg 8:394, 1992 52. Anduze AL: Merest scleral technique for primary pterygium surgery. Ophthalmic Surg 20:892, 1989 53. Desmarres LA: In Traité Théorique et Pratique des Maladies
des Yeux, 2nd ed, vol 2, p 160. Paris, Baill<age>re, 1855 54. Stocker FW: Operation for removal of pterygium. Arch Ophthalmol 27:925, 1942 55. Wilson SE, Bourne WM: Conjunctival z-plasty in the treatment of pterygium. Am J Ophthalmol 106:355, 1988 56. Kenyon KR, Wagoner MD, Hettinger ME: Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 92:1461, 1985 57. Starck T, Kenyon KR, Serrano R: Conjunctival autograft for primary and recurrent pterygia: Surgical technique
and problem management. Cornea 10:196, 1991 58. Shaw EL: A modified technique for conjunctival transplant. CLAO J 18:112, 1992 59. Friedenwald JS, Buschke W: Some factors concerned in the mitotic and wound healing activities of the
corneal epithelium. Trans Am Ophthalmol Soc 42:371, 1944 60. Davanger M, Evensen A: Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 229:560, 1971 61. Buck RC: Measurement of centripetal migration of normal corneal epithelial cells
in the mouse. Invest Ophthalmol Vis Sci 26:1296, 1985 62. Schermer A, Galvin S, Sun T-T: Differentiation-related expression of a major 64K corneal keratin in vivo
and in culture suggests a limbal location of corneal epithelial stem
cells. J Cell Biol 103:49, 1986 63. Ebato B, Friend J, Thoft R: Comparison of limbal and peripheral human corneal epithelium in tissue
culture. Invest Ophthalmol Vis Sci 28(suppl):160, 1987 64. Lavker RM, Cotsarelis G, Dong G et al: Limbal location of corneal epithelial
stem cells. In Cavanagh HD (ed): The Cornea: Transactions of the
World Congress on the Cornea III, p 23. New York, Raven Press, 1988 65. Tseng SCG: Concept and application of limbal stem cells. Eye 3:141, 1989 66. Rivaud C, Vingtain P, Cozette P et al: Autografts in pterygium surgery: Techniques
and results. J Fr Ophthalmol 9:217, 1986 (in French with English
abstract) 67. Moore TE: Keratoplasty. In Leibowitz HM (ed): Corneal Disorders, p 530. Philadelphia, WB
Saunders, 1984 68. Poirer RH, Fish JR: Lamellar keratoplasty for recurrent pterygium. Ophthalmic Surg 7:38, 1976 69. Pierse D, Casey TA: Lamellar keratoplasty. Br J Ophthalmol 43:733, 1959 70. Pearlman G, Susal AL, Hushaw J, Bartlett RE: Recurrent pterygium and treatment with lamellar keratoplasty with presentation
of a technique to limit recurrences. Ann Ophthalmol 2:763, 1970 71. Laughrea PA, Arensten JJ: Lamellar keratoplasty in the management of recurrent pterygium. Ophthalmic Surg 17:106, 1986 72. Busin M, Haliday BL, Arffa RC et al: Precarved lyophilized tissue for lamellar keratoplasty in recurrent pterygium. Am J Ophthalmol 102:222, 1986 73. Insler MS, Caldwell DR, Leach DH: Pterygium. In Brightbill FS (ed): Corneal
Surgery, p 336. St. Louis, CV Mosby, 1993 74. Krag S, Ehlers N: Excimer laser treatment of pterygium. Acta Ophthalmol 70:530, 1992 75. Cassady JR: The inhibition of pterygium recurrence by thiotepa. Am J Ophthalmol 61:886, 1966 76. Crum R, Szabo S, Folkman J: A new class of steroids inhibits angiogenesis in the presence of heparin
or a heparin fragment. Science 230:1375, 1985 77. Zauberman H: Pterygium and its recurrence. Am J Ophthalmol 63:1780, 1967 78. Meacham R: Triethylene phosphoramide in the prevention of recurrent pterygium. Am J Ophthalmol 54:751, 1962 79. Mori S: A new treatment of pterygium. Acta Soc Ophthalmol Jpn 66:990, 1962 80. Liddy BSL, Morgan JF: Triethylene thiophosphoramide (thio-tepa) and pterygium. Am J Ophthalmol 61:888, 1966 81. Chapman-Smith JS: Pterygium treatment with triethylene thiophosphoramide. Aust NZ J Ophthalmol 20:129, 1992 82. Farrel PLR, Smith RE: Bacterial corneoscleritis complicating pterygium excision. Am J Ophthalmol 107:515, 1989 83. Kleis W, Pico G: Thio-tepa therapy to prevent postoperative pterygium occurrence and neovascularization. Am J Ophthalmol 76:371, 1973 84. Asregadoo ER: Surgery, thio-tepa, and corticosteroid in the treatment of pterygium. Am J Ophthalmol 74:960, 1972 85. Ehrlich D: The management of pterygium. Ophthalmic Surg 8(2):23, 1977 86. Olander K, Kaik KG, Haik G: Management of pterygia. Should thiotepa be used?Ann Ophthalmol 10:853, 1978 87. Singh G, Wilson MR, Foster CS: Mitomycin eye drops as treatment for pterygium. Ophthalmology 95:813, 1988 88. Thoft RA: Discussion of: Mitomycin eye drops as treatment for pterygium. Ophthalmology 95:820, 1988 89. Kunimoto N, Mori S: Studies on the pterygium: IV. A treatment of the pterygium
by mitomycin C instillation. Acta Soc Ophthalmol Jpn 67:601, 1963 (English
abstract) 90. Hayasaka S, Noda S, Yamamoto Y et al: Postoperative instillation of low-dose mitomycin C in the treatment of
primary pterygium. Am J Ophthalmol 106:715, 1988 91. Lawtiantong T, Kantha V: Mitomycin-C in pterygium. Ramathibodi Med J 7:23, 1984 92. Hayasaka S, Noda S, Yamamoto Y et al: Postoperative instillation of mitomycin C in the treatment of recurrent
pterygium. Ophthalmic Surg 20:580, 1989 93. Chayakul V: Prevention of recurrent pterygium by mitomycin-C. Fortschr Ophthalmol 84:422, 1987 94. Yamanouchi U, Mishima K: Eye lesions due to mitomycin-C instillation after
pterygium operation. Folia Ophthalmol Jpn 18:854, 1967 (English abstract) 95. Yamanouchi U: A case of scleral calcification due to mitomycin-C instillation
after pterygium operation. Folia Ophthalmol Jpn 29:1221, 1978 (English
abstract) 96. Yamanouchi U, Takaku I, Tsuda N et al: Scleromalacia presumably due to mitomycin C instillation after pterygium
excision. Jpn J Clin Ophthalmol 33:139, 1979 97. Fukimachi Y, Hikita N: Ocular complication following pterygium operation
and instillation of mitomycin C. Folia Ophthalmol Jpn 32:197, 1981 (English
abstract) 98. Ewing-Chou DA, Romanchuk KG, Gilmour GR et al: Corneal melting after pterygium removal followed by topical mitomycin C
therapy. Can J Ophthalmol 27:197, 1992 99. Rubenfeld RS, Pfister RR, Stein RM et al: Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology 99:1647, 1992 100. Gupta S, Basti S: Corneoscleral, ciliary body, and vitreoretinal toxicity after excessive
instillation of mitomycin C. Am J Ophthalmol 114:503, 1992 101. Peyman GA, Greenberg D, Fishman GA et al: Evaluation of toxicity of intravitreal antineoplastic drugs. Ophthalmic Surg 15:411, 1984 102. Derick RJ, Pasquale L, Quigley HA, Jampel H: Potential toxicity of mitomycin C. Arch Ophthalmol 109:1635, 1991 103. Folkman J: Tumor angiogenesis: Therapeutic implications. N Engl J Med 285:1182, 1971 104. Denekamp J: Vasculature as a target for tumour therapy. In Messmer K, Hammersen
F (eds): Progress in Applied Microcirculation, p 28. Basel, Karger, 1984 105. Burnam CF, Neill W: Use of beta ray of radium applicator. South Med J 33:279, 1940 106. Nowell JF: Management of pterygia 20 years later. South Med J 79:1382, 1986 107. Pinkerton OD: Strontium barriers in pterygium surgery. Trans Am Acad Ophthalmol Otolaryngol 79:613, 1975 108. Alaniz-Camino F: The use of postoperative beta radiation in the treatment of pterygia. Ophthalmic Surg 13:1022, 1982 109. Bahrassa F, Datta R: Postoperative beta radiation treatment of pterygium. Int J Radiat Oncol Biol Phys 9:679, 1983 110. de Keizer RJW, Swart-Van den Berg M, Baartse WJ: Results of pterygium excision with Sr 90 irradiation, lamellar keratoplasty, and
conjunctival flaps. Doc Ophthalmol 67:33, 1987 111. Hilgers JHC: Strontium 90 ß-irradiation, cataractogenicity, and pterygium recurrence. Arch Ophthalmol 76:329, 1966 112. Tarr KH, Constable IJ: Late complications of pterygium treatment. Br J Ophthalmol 64:496, 1980 113. MacKenzie FD, Hirst LW, Kynaston B, Bain C: Recurrence rate and complications after beta irradiation for pterygia. Ophthalmology 98:1776, 1991 114. van den Brenk HAS: Results of prophylactic postoperative irradiation in 1300 cases of pterygium. AJR 103:723, 1968 115. Friedell HL, Thomas CI, Krohmer JS: Beta-ray application to the eye. Am J Ophthalmol 33:525, 1950 116. Aswad MI, Baum J: Optimal time for postoperative irradiation of pterygia. Ophthalmology 94:1450, 1987 117. Robert Y, Pauli L, Gysin P et al: Protracted ruthenium treatment of recurrent pterygium. Graefes Arch Clin Exp Ophthalmol 230:233, 1992 118. Merriam GR: The effects of beta radiation on the eye. Radiology 66:240, 1956 119. Thomas CI, Storaasli JP, Friedell HL: Lenticular changes associated with beta radiation of the eye and their
significance. Radiology 79:588, 1962 120. Lentino W, Zaret MM, Rossignol B et al: Treatment of pterygium by surgery followed by beta radiation: An analysis
of 256 cases. AJR 81:93, 1959 121. Hellein R: Radionecrosis of the sclera-treated with auricular cartilage graft. Aust J Ophthalmol 4:104, 1976 122. Cameron ME: Histology of pterygium: An electron microscopic study. Br J Ophthalmol 67:604, 1983 123. Levine DJ: Beta irradiation of pterygium. Ophthalmology 99:841, 1992 124. Cameron ME: Preventable complications of pterygium excision with beta irradiation. Br J Ophthalmol 56:52, 1972 125. Margo CE, Polack FM, Mood CI: Aspergillus panophthalmitis complicating treatment of pterygium. Cornea 7:285, 1988 |