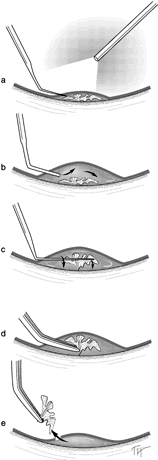
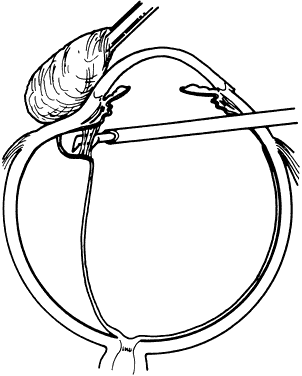
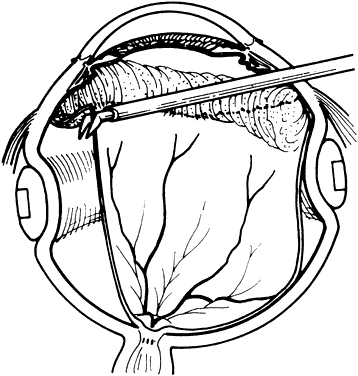
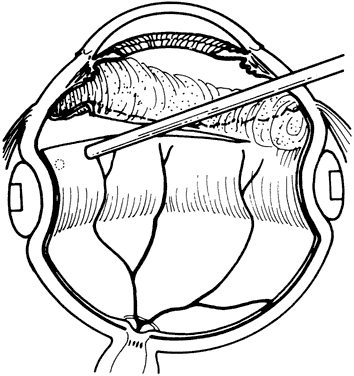
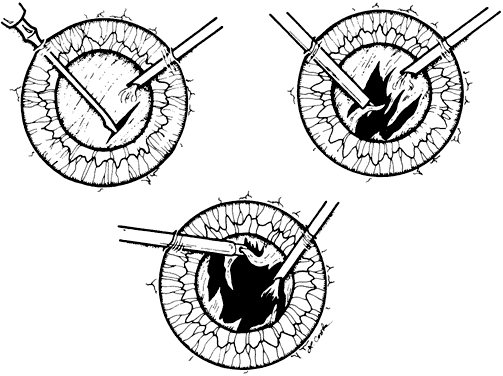
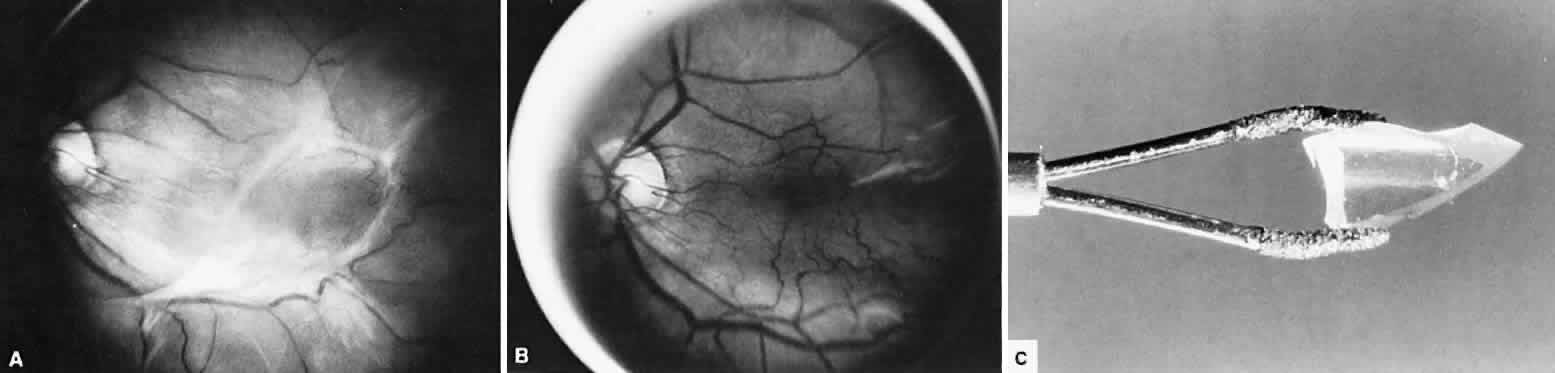
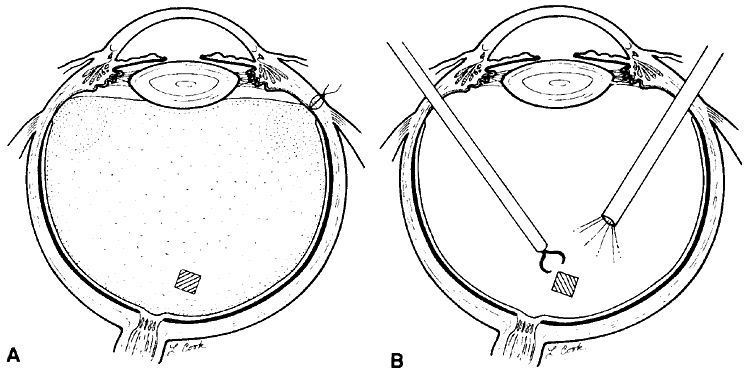
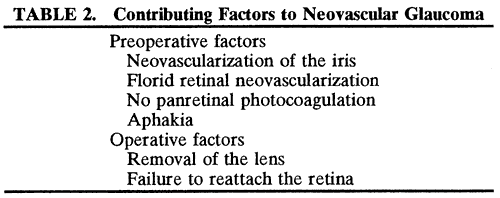
1. Kasner D: A new approach to the management of vitreous. Highlights Ophthalmol 11:304–329, 1968 2. Machemer R et al: Vitrectomy: A pars plana approach. Trans Am Acad Ophthalmol Otolaryngol 75:813–820, 1971 3. Machemer R, Parel JM, Buettner H: A new concept for vitreous surgery. I. Instrumentation. Am J Ophthalmol 74:1022–1033, 1972 4. Machemer R: Development of pars plana vitrectomy: My personal contribution. Klin Monatsbl Augenheilkd 207:147–161, 1995 5. Parel JM, Machemer R, Aumayr W: A new concept for vitreous surgery. IV. Improvements in instrumentation
and illumination. Am J Ophthalmol 77:6–12, 1974 6. Machemer R: A new concept of vitreous surgery. II. Surgical technique and complications. Am J Ophthalmol 74:1022–1033, 1972 7. O'Malley C, Heintz R: Vitrectomy with an alternative instrument system. Ann Ophthalmol 7:585–594, 1975 8. Williams GA, Abrams GW, Mieler WF: Illuminated retinal picks for vitreous surgery. Arch Ophthalmol 107:1086, 1989 9. Kuhn F, Mester V, Berta A: The Tano Diamond Dusted Membrane Scraper: indications
and contraindications. Acta Ophthalmol Scand 76:754–755, 1998 (letter) 10. Lewis JM et al: Diamond-dusted silicone cannula for epiretinal membrane separation during
vitreous surgery. Am J Ophthalmol 124:553–554, 1997 11. Benson W, Diamond J, Tasman W: Intraocular irrigating solutions for pars plana vitrectomy. A prospective
randomized, double-blind study. Arch Ophthalmol 99:1013, 1981 12. Edelhauser HF, Gonnering R, Van Horn D: Intraocular irrigating solutions: A comparative study of BSS plus and lactated
Ringer's solution. Arch Ophthalmol 96:516, 1978 13. Matsuda M, Tano Y, Edelhauser HF: Comparison of intra-ocular irrigating solutions used for pars plana vitrectomy
and prevention of endothelial cell loss. Jpn J Ophthalmol 28:230–238, 1984 14. DeJuan E, Hickingbotham D: Flexible iris retractor. Am J Ophthalmol 111:776–777, 1991 15. Chang S: Low viscosity liquid fluorochemicals in vitreous surgery. Am J Ophthalmol 103:38–43, 1987 16. Cibis P et al: The use of liquid silicone in retinal detachment surgery. Arch Ophthalmol 68:590–599, 1962 17. Capone A, Aaberg TM: Silicone oil in vitreoretinal surgery. Curr Opin Ophthalmol 6:33–37, 1995 18. Spitznas M, Reiner J: A stereoscopic diagonal inverter (SDI) for wide-angle vitreous surgery. Graefes Arch Clin Exp Ophthalmol 225:9–12, 1987 19. Spitznas M: A binocular indirect ophthalmomicroscope (BIOM) for non-contact wide-angle
vitreous surgery. Graefes Arch Clin Exp Ophthalmol 225:13–15, 1987 20. Summanen P, Karhunen U, Laatikainen L: Characteristics and survival of diabetic patients undergoing vitreous surgery. Acta Ophthalmol (Copenh) 65:197–202, 1987 21. Blankenship G: Preoperative prognostic factors in diabetic pars plana vitrectomy. Ophthalmology 89:1246–1249, 1982 22. Ryan E, Gilbert HD: Results of surgical treatment of recent-onset full thickness idiopathic
holes. Arch Ophthalmol 112:1545–1553, 1994 23. Rice TA et al: Prognostic factors in vitrectomy for epiretinal membranes of the macula. Ophthalmology 93:602–610, 1986 24. Scherfig E et al: Prognostic parameters in pars plana vitrectomy. Acta Ophthalmol (Copenh) 61:788–805, 1983 25. Landers MB et al: Temporary keratoprosthesis for use during pars plana vitrectomy. Am J Ophthalmol 91:615–619, 1981 26. Eckardt C: A new temporary keratoprosthesis for pars plana vitrectomy. Retina 7:34–37, 1987 27. Eckardt C: A new temporary keratoprosthesis. Klin Monatsbl Augenheilkd 191:243, 1987 28. de Bustros S et al: Vitrectomy for progressive proliferative diabetic retinopathy. Arch Ophthalmol 105:196–199, 1987 29. Thompson JT et al: Prognostic indicators of success and failure in vitrectomy for diabetic
retinopathy. Ophthalmology 93:290–295, 1986 30. Thompson JT et al: Results and prognostic factors in vitrectomy for diabetic
vitreous hemorrhage. Arch Ophthalmol 105:191–195, 1987 (published
erratum appears in Arch Ophthalmol 105:496, 1987) 31. Benson WE et al: Extracapsular cataract extraction, posterior chamber lens insertion, and
pars plana vitrectomy in one operation. Ophthalmology 97:918–921, 1990 32. Koenig SB et al: Combined phacoemulsification, pars plana vitrectomy, and posterior chamber
intraocular lens insertion. Arch Ophthalmol 110:1101–1104, 1992 33. Kokame GT, Flynn Jr HW, Blankenship GW: Posterior chamber intraocular lens implantation during diabetic pars plana
vitrectomy. Ophthalmology 96:603–610, 1989 34. Mamalis N et al: Phacoemulsification combined with pars plana vitrectomy. Ophthalmic Surg 22:194–198, 1991 35. McElvanney AM, Talbot EM: Posterior chamber lens implantation combined with pars plana vitrectomy. J Cataract Refract Surg 23:106–110, 1997 36. Menchini U et al: Combined vitrectomy, cataract extraction, and posterior chamber intraocular
lens implantation in diabetic patients. Ophthalmic Surg 22:69–73, 1991 37. Smiddy WE: Intraocular lens insertion during vitrectomy. Ophthalmic Surg 23:808–810, 1992 38. Senn P, Schipper I, Perren B: Combined pars plana vitrectomy, phacoemulsification, and intraocular lens
implantation in the capsular bag: A comparison to vitrectomy and subsequent
cataract surgery as a two-step procedure. Ophthalmic Surg Lasers 26:420–428, 1995 39. Francese J et al: Moisture droplet formation on the posterior surface of intraocular lenses
during fluid/air exchange. J Cataract Refract Surg 21:685–689, 1995 40. Hainsworth DP et al: Condensation on polymethylmethacrylate, acrylic polymer, and silicone intraocular
lenses after fluid-air exchange in rabbits. Ophthalmology 103:1410–1418, 1996 41. Coleman D, Abramson D: Ocular ultrasonography. In: Duane T (ed): Clinical
Ophthalmology. Hagerstown, MD: Harper & Row, 1990 42. Rubsamen PE et al: Diagnostic ultrasound and pars plana vitrectomy in penetrating ocular trauma. Ophthalmology 101:809–814, 1994 43. Hotta K et al: Ultrasound biomicroscopy for examination of the sclerotomy site in eyes
with proliferative diabetic retinopathy after vitrectomy. Retina 20:52–58, 2000 44. Boker T, Spitznas M: Ultrasound biomicroscopy for examination of the sclerotomy site after pars
plana vitrectomy. Am J Ophthalmol 118:813–815, 1994 45. Mandelcorn M, Blankenship G, Machemer R: Pars plana vitrectomy in severe diabetic retinopathy. Am J Ophthalmol 57:530–542, 1976 46. Sinclair S, Loebl M, Riva C: Blue field entopic test in patients with ocular trauma. Arch Ophthalmol 99:464–467, 1981 47. Algvere P, Persson HE, Wanger P: Preoperative electroretinograms and visual evoked cortical potentials for
predicting outcome of vitrectomy in diabetics. Retina 5:179–183, 1985 48. Summanen P: Vitrectomy for diabetic eye disease. The prognostic value of pre-operative electroretinography and visual evoked
cortical potentials. Ophthalmologica 199:60–71, 1989 49. Terasaki H et al: Focal macular electroretinograms before and after successful macular hole
surgery. Am J Ophthalmol 125:204–213, 1998 50. Terasaki H et al: Focal macular electroretinogram before and after drainage of macular subretinal
hemorrhage. Am J Ophthalmol 123:207–211, 1997 51. Gribomont AC, Guerit JM: Prognostic value of preoperative flash and pattern visual evoked potentials
for diabetic retinopathy vitrectomy. Eur J Ophthalmol 4:218–222, 1994 52. Scherfig E et al: Flash visual evoked potential as a prognostic factor for vitreous operations
in diabetic eyes. Ophthalmology 91:1475–1479, 1984 53. Bryden F, Pyott A, McGhee C: Real time ultrasound in the assessment of intraocular foreign bodies. Eye 4:727–731, 1990 54. Kuhn F, Morris R: Posterior segment intraocular foreign bodies: Management
in the vitrectomy era. Ophthalmology 107:821–822, 2000 (editorial) 55. Chacko J et al: Detection and localization of steel intraocular foreign bodies using computed
tomography. Ophthalmology 104:319–323, 1997 56. Puliafito CA et al: Optical Coherence Tomography of Ocular Diseases. Thorofare, NJ: Slack, 1996 57. Gobel W et al: Findings of optical coherence tomography (OPT) before and after macular
hole surgery. Ophthalmologe 97:251–256, 2000 58. Munuera JM et al: Optical coherence tomography in successful surgery of
vitreomacular traction syndrome. Arch Ophthalmol 116:1388–1389, 1998 (letter) 59. Hee M, Puliafito CA, Wong C: Optical coherence tomography of macular holes. Ophthalmology 102:748–756, 1995 60. Hee M et al: Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol 113:1019–1029, 1995 61. Mikajiri K et al: Analysis of vitrectomy for idiopathic macular hole by optical coherence
tomography. Am J Ophthalmol 128:655–657, 1999 62. Otani T, Kishi S: Tomographic assessment of vitreous surgery for diabetic macular edema. Am J Ophthalmol 129:487–494, 2000 63. Tripathi R et al: Fundamentals and principles of ophthalmology. In: Basic
and Clinical Science Course. San Francisco: American Academy of Ophthalmology, 1999:64–65 64. Sebag J: The Vitreous: Structure, Function, and Pathobiology. New York: Springer-Verlag, 1989 65. Gass JD: Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. St
Louis: Mosby, 1997:904–909 66. Calenda E et al: Peribulbar anesthesia and sub-Tenon injection for vitreoretinal surgery: 300 cases. Acta Ophthalmol Scand 78:196–199, 2000 67. Brucker AJ, Saran BR, Maguire AM: Perilimbal anesthesia for pars plana
vitrectomy. Am J Ophthalmol 117:599–602, 1994 (published erratum
appears in Am J Ophthalmol 118:273, 1994) 68. Nicholson AD et al: Peribulbar anesthesia for primary vitreoretinal surgery. Ophthalmic Surg 23:657–661, 1992 69. Li HK et al: Sub-tenon's injection for local anesthesia in posterior
segment surgery. Ophthalmology 107:41–46, 2000; discussion, 46–47 70. Friedberg MA et al: An alternative technique of local anesthesia for vitreoretinal surgery. Arch Ophthalmol 109:1615–1616, 1991 71. Sharma T et al: Parabulbar anesthesia for primary vitreoretinal surgery. Ophthalmology 104:425–428, 1997 72. Yepez J, Cedeno de Yepez J, Arevalo JF: Topical anesthesia in posterior vitrectomy. Retina 20:41–45, 2000 73. Yepez J, Cedeno de Yepez J, Arevalo JF: Topical anesthesia for phacoemulsification, intraocular lens implantation, and
posterior vitrectomy. J Cataract Refract Surg 25:1161–1164, 1999 74. Haimann MH, Abrams GW: Prevention of lens opacification during diabetic vitrectomy. Ophthalmology 91:116–121, 1984 75. Landers D, Stefansson E, Wolbarsht M: The optics of vitreous surgery. Am J Ophthalmol 91:611–614, 1981 76. Lesnoni G et al: The use of panoramic viewing system in relaxing retinotomy and retinectomy. Retina 17:186–190, 1997 77. Virata SR, Kylstra JA, Singh HT: Corneal epithelial defects following vitrectomy surgery using hand-held, sew-on, and
noncontact viewing lenses. Retina 19:287–290, 1999 78. Charles S: Vitreous surgery. In Ryan SJ (ed): Retina. St Louis: CV Mosby, 1989:193 79. DeJuan E, Landers MI: New techniques for visualization of infusion cannula during vitreous surgery. Am J Ophthalmol 97:657, 1984 80. Welch JC, Spaeth GL, Benson W: Massive suprachoroidal hemorrhage. Ophthalmology 95:1202, 1988 81. Rice TA et al: The effect of lensectomy on the incidence of iris neovascularization and
neovascular glaucoma after vitrectomy for diabetic retinopathy. Am J Ophthalmol 95:1–11, 1983 82. Ryan EH Jr, Gilbert HD: Lensectomy, vitrectomy indications, and techniques in cataract surgery. Curr Opin Ophthalmol 7:69–74, 1996 83. Smiddy W: Dislocated posterior chamber intraocular lens, a new technique of management. Arch Ophthalmol 107:1678–1680, 1989 84. Campo RV, Chung KD, Oyakawa RT: Pars plana vitrectomy in the management of dislocated posterior chamber
lenses. Am J Ophthalmol 108:529–534, 1989 85. Wallace RT et al: The use of perfluorophenanthrene in the removal of intravitreal lens fragments. Am J Ophthalmol 116:196–200, 1993 86. Shapiro MJ et al: Management of the dislocated crystalline lens with a perfluorocarbon liquid. Am J Ophthalmol 112:401–405, 1991 87. Flynn HW, Brod RD: Use of a soft-tipped extrusion needle for epimacular membrane peeling. Arch Ophthalmol 108:20–21, 1990 88. Mester V, Kuhn F: Internal limiting membrane removal in the management of full-thickness
macular holes. Am J Ophthalmol 129:769–777, 2000 89. Moisseiev J, Glaser BM: New and previously unidentified retinal breaks in eyes with recurrent retinal
detachment with proliferative vitreoretinopathy. Arch Ophthalmol 107:1152, 1989 90. McLeod D, James C: Viscodelamination at the vitreoretinal junction in severe diabetic eye
disease. Br J Ophthalmol 72:413–419, 1988 91. Ryan E: An illuminated cannula for viscodelamination. Arch Ophthalmol 107:1085, 1989 92. Abrams GW, Williams GA: “En bloc” excision of diabetic membranes. Am J Ophthalmol 103(3 Pt 1):302–308, 1987 93. Han DP, Murphy ML, Mieler WF: A modified en bloc excision technique during vitrectomy for diabetic traction
retinal detachment. Results and complications. Ophthalmology 101:803–808, 1994 94. Meier P, Wiedemann P: Vitrectomy for traction macular detachment in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 235:569–574, 1997 95. Williams DF et al: Results of vitrectomy for diabetic traction retinal detachments using the
en bloc excision technique. Ophthalmology 96:752–758, 1989 96. Charles S: Vitrectomy for retinal detachment. Trans Ophthalmol Soc UK 100:542–549, 1980 97. Machemer R: Surgical approaches to subretinal bands. Am J Ophthalmol 90:81–85, 1980 98. Steinmetz RL, Masket S, Sidikaro Y: The successful removal of a subretinal cysticercus by pars plana vitrectomy. Retina 9:276–280, 1989 99. Natarajan S et al: Management of intraocular cysticercosis. Graefes Arch Clin Exp Ophthalmol 237:812–814, 1999 100. Wade EC et al: Subretinal hemorrhage management by pars plana vitrectomy and internal
drainage. Arch Ophthalmol 108:973–978, 1990 101. Zhao P et al: Vitrectomy for macular hemorrhage associated with retinal arterial macroaneurysm. Ophthalmology 107:613–617, 2000 102. Lim JI et al: Submacular hemorrhage removal. Ophthalmology 102:1393–1399, 1995 103. Lewis H: Intraoperative fibrinolysis of submacular hemorrhage with tissue plasminogen
activator and surgical drainage. Am J Ophthalmol 118:559–568, 1994 104. Thomas MA et al: Surgical management of subfoveal choroidal neovascularization. Ophthalmology 99:952–966, 1992 105. Berger A, Kaplan HJ: Clinical experience with the surgical removal of subfoveal neovascular
membranes. Ophthalmology 99:969–976, 1992 106. Lambert HM et al: Surgical excision of subfoveal neovascular membranes in age-related macular
degeneration. Am J Ophthalmol 114:241–242, 1992 107. Thomas MA, Kaplan HJ: Surgical removal of subfoveal neovascularization in the presumed ocular
histoplasmosis syndrome. Am J Ophthalmol 111:1–7, 1991 108. Machemer R: Retinotomy. Am J Ophthalmol 92:768, 1981 109. Machemer R, McCuen B, DeJuan E: Relaxing retinotomies and retinectomies. Am J Ophthalmol 102:7–12, 1986 110. Aaberg TM: Management of anterior and posterior proliferative vitreoretinopathy. XLV
Edward Jackson Memorial Lecture. Am J Ophthalmol 106:519, 1988 111. Lewis H, Aaberg TM, Abrams GW: Causes of failure after initial vitreoretinal surgery for severe proliferative
vitreoretinopathy. Am J Ophthalmol 111:8, 1991 112. Lewis H, Aaberg TM: Causes of failure after repeat vitreoretinal surgery for recurrent proliferative
vitreoretinopathy. Am J Ophthalmol 111:15, 1991 113. Blumenkranz M et al: Relaxing retinotomy with silicone oil or long-acting gas in eyes with severe
proliferative vitreoretinopathy. Silicone Study Report 5. Am J Ophthalmol 116:557–564, 1993 114. Han DP et al: Vitrectomy for traumatic retinal incarceration. Arch Ophthalmol 106:640–645, 1988 115. Mathis A: Fluid-air exchange. J Fr Ophthalmol 15:367–371, 1992 116. Charles S: Ancillary techniques to trans pars plana vitrectomy. In McPherson
A (ed): New and Controversial Aspects of Vitreoretinal Surgery. St
Louis: CV Mosby, 1977:196–197 117. Charles S: A motorized gas injector for vitreous surgery. Arch Ophthalmol 99:1981, 1981 118. McCuen B et al: Automated fluid-gas exchange. Am J Ophthalmol 95:717, 1983 119. Hueneke RL, Aaberg TM: Instrumentation for continuous fluid-air exchange during vitreous surgery. Am J Ophthalmol 96:547–548, 1983 120. Doft BH: Intentional retinotomy for internal drainage of subretinal fluid. Arch Ophthalmol 104:807–808, 1986 121. Landers D: Sodium hyaluronate (Healon) as an aid to internal fluid-gas exchange. Am J Ophthalmol 94:557–558, 1982 122. Friberg TR: Clinical experience with a binocular indirect ophthalmoscope laser delivery
system. Retina 7:28–31, 1987 123. Campochiaro PA et al: Cryotherapy enhances intravitreal dispersion of viable retinal pigment
epithelial cells. Arch Ophthalmol 103:434–436, 1985 124. Bishara S, Buzney SM: Dispersion of retinal pigment epithelial cells from experimental retinal
holes. Arch Ophthalmol 229:195–199, 1991 125. Singh A, Michels R, Glaser BM: Scleral indentation following cryotherapy and repeat cryotherapy enhance
release of viable retinal pigment epithelial cells. Retina 6:176–178, 1986 126. Peyman GA, Grisolano J, Palacio M: Intraocular photocoagulation with argon-krypton laser. Arch Ophthalmol 98:2062–2064, 1980 127. Chang S: Perfluorocarbon liquids in vitreoretinal surgery. Int Ophthalmol Clin 32:153–163, 1992 128. Peyman GA, Schulman J, Sullivan B: Perfluorocarbon liquids in ophthalmology. Surv Ophthalmol 39:375–395, 1995 129. Loewenstein A et al: Perfluoroperhydrophenanthrene versus perfluoro-n-octane in vitreoretinal surgery. Ophthalmology 107:1078–1082, 2000 130. Scott IU et al: Outcomes and complications associated with perfluoro-n-octane and perfluorperhydrophenanthrene in complex retinal detachment
repair. Ophthalmology 107:860–865, 2000 131. Rosengren B: Results of treatment of detachment of the retina with diathermy and injection
of air into the vitreous. Arch Ophthalmol 16:573–579, 1938 132. Cekic O, Ohji M: Intraocular gas tamponades. Semin Ophthalmol 15:3–14, 2000 133. Chang S: Vitreous substitutes. In Guyer D et al (eds): Retina, Vitreous, Macula. Philadelphia: WB
Saunders, 1999:1320–1327 134. DeJuan E, McCuen B, Tiedeman J: Intraocular tamponade and surface tension. Surv Ophthalmol 30:47–51, 1985 135. Thompson JT: Kinetics of intraocular gases. Arch Ophthalmol 107:687–691, 1989 136. Williams D et al: A two-stage technique for intraoperative fluid-gas exchange following pars
plana vitrectomy. Arch Ophthalmol 108:1484, 1990 137. Cibis P et al: The use of silicone oil in retinal detachment surgery. Arch Ophthalmol 68:590–599, 1962 138. Yamamoto S, Takeuchi S: Silicone oil and fluorosilicone. Semin Ophthalmol 15:15–24, 2000 139. Regillo C et al: Repair of retinitis-related retinal detachments with silicone oil in patients
with acquired immunodeficiency syndrome. Am J Ophthalmol 113:21–27, 1992 140. Lim JI et al: Improved visual results after surgical repair of cytomegalovirus-related
retinal detachments. Ophthalmology 101:264–269, 1994 141. Azen SP et al: Silicone oil in the repair of complex retinal detachments: A prospective
observational multicenter study. Ophthalmology 105:1587–1597, 1998 142. Barr CC et al: Postoperative intraocular pressure abnormalities in the Silicone Study. Silicone
Study Report 4. Ophthalmology 100:1629–1635, 1993 143. Lewis H et al: Perisilicone proliferation after vitrectomy for proliferative vitreoretinopathy. Ophthalmology 95:583–591, 1988 144. Laroche L et al: Ocular findings following intravitreal silicone oil injection. Arch Ophthalmol 101:1422–1425, 1983 145. Ni C et al: Intravenous silicone injection. Arch Ophthalmol 101:1399–1401, 1983 146. Parmley V et al: Foreign-body giant cell reaction to liquid silicone. Am J Ophthalmol 101:680–683, 1986 147. Gonvers M, Hornung J, de Courten C: The effect of liquid silicone on the rabbit retina. Arch Ophthalmol 104:1057–1062, 1986 148. Chan CC, Okun E: The question of ocular tolerance to intravitreal liquid silicone: A long-term
analysis. Ophthalmology 93:651–659, 1986 149. Federman J, Schubert HD: Complications associated with the use of silicone oil in 150 eyes after
retina-vitreous surgery. Ophthalmology 95:870–876, 1988 150. Brourman N, Blumenkranz M, Cox MS: Silicone oil for the treatment of severe proliferative diabetic retinopathy. Ophthalmology 96:759–764, 1989 151. McCuen BW, Rinkoff JS: Silicone oil for progressive anterior ocular neovascularization
after failed diabetic vitrectomy. Arch Ophthalmol 107:677–682, 1989 (published erratum appears in Arch Ophthalmol 107:1030, 1989) 152. Gonvers M: Temporary silicone oil tamponade in the management of retinal detachment
with proliferative vitreoretinopathy. Am J Ophthalmol 100:239–245, 1985 153. Cox MS, Trese MT, Murphy P: Silicone oil for advanced proliferative vitreoretinopathy. Ophthalmology 93:646–650, 1986 154. McCuen B et al: Silicone oil in vitreoretinal surgery. Part 2: Results and complications. Retina 5:198–205, 1985 155. McCuen BW, Landers MB, Machemer R: The use of silicone oil following failed vitrectomy for retinal detachment
with advanced proliferative vitreoretinopathy. Ophthalmology 92:1029–1034, 1985 156. Kirchhof B: The contribution of vitreoretinal surgery to the management of refractory
glaucomas. Curr Opin Ophthalmol 10:117–120, 1999 157. Abu el-Asrar AM, al-Obeidan SA: Pars plana vitrectomy in the management of ghost cell glaucoma. Int Ophthalmol 19:121–124, 1995 158. Brucker AJ, Michels R, Green WR: Pars plana vitrectomy in the management of blood-induced glaucoma with
vitreous hemorrhage. Ann Ophthalmol 10:1427–1437, 1978 159. Epstein DL: The malignant glaucoma syndromes. In Epstein DL, Allingham
R, Schuman JS (eds): Chandler and Grant's Glaucoma. Baltimore: Williams & Wilkins, 1997:285–303 160. Harbour JW, Rubsamen PE, Palmberg P: Pars plana vitrectomy in the management of phakic and pseudophakic malignant
glaucoma. Arch Ophthalmol 114:1073–1078, 1996 161. Lynch MG et al: Surgical vitrectomy for pseudophakic malignant glaucoma. Am J Ophthalmol 102:149–153, 1986 162. Gandham SB et al: Aqueous tube-shunt implantation and pars plana vitrectomy in eyes with
refractory glaucoma. Am J Ophthalmol 116:189–195, 1993 163. Luttrull JK, Avery RL: Pars plana implant and vitrectomy for treatment of neovascular glaucoma. Retina 15:379–387, 1995 164. Scott IU et al: Combined pars plana vitrectomy and glaucoma drainage implant placement
for refractory glaucoma. Am J Ophthalmol 129:334–341, 2000 165. Varma R et al: Pars plana Baerveldt tube insertion with vitrectomy in glaucomas associated
with pseudophakia and aphakia. Am J Ophthalmol 119:401–407, 1995 166. Luttrull JK et al: Initial experience with pneumatically stented Baerveldt implant modified
for pars plana insertion for complicated glaucoma. Ophthalmology 107:143–150, 2000 167. Wallace RT et al: The use of perfluorophenanthrene in the removal of intravitreal lens fragments. Am J Ophthalmol 116:196–200, 1993 168. Mello M et al: Surgical management and outcomes of dislocated intraocular lenses. Ophthalmology 107:62–67, 2000 169. Lambrou FH, Stewart M: Management of dislocated lens fragments during phacoemulsification. Ophthalmology 99:1260–1262, 1992 170. Kapusta MA, Chen CJ, Lam WC: Outcomes of dropped nucleus during phacoemulsification. Ophthalmology 103:1184–1187, 1996 171. Fastenberg DM et al: Management of dislocated nuclear fragments after phacoemulsification. Am J Ophthalmol 112:535–539, 1991 172. Blodi BA et al: Retained nuclei after cataract surgery. Ophthalmology 99:41–44, 1992 173. Margherio RR et al: Vitrectomy for retained lens fragments after phacoemulsification. Ophthalmology 104:1426–1432, 1997 174. Kim JE et al: Retained lens fragments after phacoemulsification. Ophthalmology 101:1827–1832, 1994 175. Gilliland GD, Hutton WL, Fuller DG: Retained intravitreal lens fragments
after cataract surgery. Ophthalmology 99:1263–1267, 1992; discussion, 1268–1269 176. Borne MJ et al: Outcomes of vitrectomy for retained lens fragments. Ophthalmology 103:971–976, 1996 177. Vilar NF et al: Removal of retained lens fragments after phacoemulsification
reverses secondary glaucoma and restores visual acuity. Ophthalmology 104:787–791, 1997; discussion, 791–792 178. Diabetic Retinopathy Vitrectomy Study Research Group: Two-year course of
visual acuity in severe proliferative diabetic retinopathy with conventional
management. Diabetic Retinopathy Vitrectomy Study (DRVS) Report 1. Ophthalmology 92:492–502, 1985 179. Diabetic Retinopathy Vitrectomy Study Research Group: Early vitrectomy
for severe vitreous hemorrhage in diabetic retinopathy. Two-year results
of a randomized trial. Diabetic Retinopathy Vitrectomy Study Report 2. Arch
Ophthalmol 103:1644–1652, 1985 180. Diabetic Retinopathy Vitrectomy Study Research Group: Early vitrectomy
for severe vitreous hemorrhage in diabetic retinopathy: Four-year results
of a randomized trial. Diabetic Retinopathy Vitrectomy Study Report 5. Arch
Ophthalmol 108:958–964, 1990 (published erratum appears
in Arch Ophthalmol 108:1452, Oct 1990) 181. Benson WE et al: Complications of vitrectomy for non-clearing vitreous hemorrhage in diabetic
patients. Ophthalmic Surg 19:862–864, 1988 182. Blankenship GW, Machemer R: Long-term diabetic vitrectomy results: Report of 10 year follow-up. Ophthalmology 92:503–506, 1985 183. Ramsay RC, Knobloch WH, Cantrill HL: Timing of vitrectomy for active proliferative diabetic retinopathy. Ophthalmology 93:283–289, 1986 184. O'Hanley GP, Canny CL: Diabetic dense premacular hemorrhage: A possible indication for prompt
vitrectomy. Ophthalmology 92:507–511, 1985 185. Packer AJ: Vitrectomy for progressive macular traction associated with proliferative
diabetic retinopathy. Arch Ophthalmol 105:1679–1682, 1987 186. Charles S, Flinn C: The natural history of diabetic extramacular traction retinal detachment. Arch Ophthalmol 99:66–68, 1981 187. Thompson JT et al: Results and prognostic factors in vitrectomy for diabetic traction-rhegmatogenous
retinal detachment. Arch Ophthalmol 105:503–507, 1987 188. Rice TA, Michels R, Rice E: Vitrectomy for diabetic rhegmatogenous retinal detachment. Am J Ophthalmol 95:34–44, 1983 189. Yeo JH, Glaser BM, Michels R: Silicone oil in the treatment of complicated retinal detachments. Ophthalmology 94:94, 1987 190. Sato Y et al: Vitrectomy for diabetic macular heterotopia. Ophthalmology 101:63–67, 1994 191. Bresnick G, Haight B, de Venecia G: Retinal wrinkling and macular heterotopia in diabetic retinopathy. Arch Ophthalmol 97:1890, 1979 192. Diabetic Retinopathy Vitrectomy Study Research Group: Early vitrectomy
for severe proliferative diabetic retinopathy in eyes with useful vision: Results
of a randomized trial. Diabetic Retinopathy Vitrectomy Study
Report 3. Ophthalmology 95:1307–1320, 1988 193. Diabetic Retinopathy Vitrectomy Study Research Group: Early vitrectomy
for severe proliferative diabetic retinopathy in eyes with useful vision: Clinical
application of results of a randomized trial, Diabetic Retinopathy
Vitrectomy Study Report 4. Ophthalmology 95:1321–1334, 1988 194. Spencer R, McMeel J, Franks E: Visual outcome in moderate and severe proliferative diabetic retinopathy. Arch Ophthalmol 99:1551, 1981 195. Favard C et al: Full panretinal photocoagulation and early vitrectomy improve prognosis
of florid diabetic retinopathy. Ophthalmology 103:561–574, 1996 196. Chaudhry NA et al: Early vitrectomy and endolaser photocoagulation in patients
with type I diabetes with severe vitreous hemorrhage. Ophthalmology 102:1164–1169, 1995 (published erratum appears in Ophthalmology 102:1740, 1995) 197. Capone A, Panozzo G: Vitrectomy for refractory diabetic macular edema. Semin Ophthalmol 15:78–80, 2000 198. Lewis H et al: Vitrectomy for diabetic macular traction and edema associated with posterior
hyaloidal traction. Ophthalmology 99:753–759, 1992 199. Harbour JW et al: Vitrectomy for diabetic macular edema associated with a thickened and taut
posterior hyaloid membrane. Am J Ophthalmol 121:405–413, 1996 200. Ikeda T et al: Vitrectomy for cystoid macular oedema with attached posterior hyaloid membrane
in patients with diabetes. Br J Ophthalmol 83:12–14, 1999 201. Tachi N, Ogino N: Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol 122:258–260, 1996 202. Brazitikos PD: The expanding role of primary pars plana vitrectomy in the treatment of
rhegmatogenous noncomplicated retinal detachment. Semin Ophthalmol 15:65–77, 2000 203. Escoffery RF et al: Vitrectomy without scleral buckling for primary rhegmatogenous retinal
detachment. Am J Ophthalmol 99:275–281, 1985 204. Heimann H et al: Primary vitrectomy without scleral buckling for rhegmatogenous retinal
detachment. Graefes Arch Clin Exp Ophthalmol 234:561–568, 1996 205. Wolfensberger TJ, Gonvers M: Long-term follow-up for retinal detachment due to macular hole in myopic
eyes treated by temporary silicone oil tamponade and laser photocoagulation. Ophthalmology 106:1786–1791, 1999 206. Verstraeten T et al: Lens-sparing vitrectomy with perfluorocarbon liquid for the primary treatment
of giant retinal tears. Ophthalmology 102:17–20, 1995 207. Campo RV et al: Pars plana vitrectomy without scleral buckle for pseudophakic
retinal detachments. Ophthalmology 106:1811–1815, 1999; discussion, 1816 208. Brazitikos PD et al: Primary vitrectomy with perfluoro-n-octane use in the treatment of pseudophakic
retinal detachment with undetected retinal breaks. Retina 19:103–109, 1999 209. Desai UR, Strassman IB: Combined pars plana vitrectomy and scleral buckling for pseudophakic and
aphakic retinal detachments in which a break is not seen preoperatively. Ophthalmic Surg Lasers 28:718–722, 1997 210. Pastor JC: Proliferative vitreoretinopathy: An overview. Surv Ophthalmol 43:3–18, 1998 211. Committee RST: The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology 90:121–125, 1983 212. Lean J et al: Classification of proliferative vitreoretinopathy used in the silicone
study. Ophthalmology 96:765–771, 1989 213. Lean J et al: The prognostic utility of the Silicone Study Classification System. Silicone
Study Report 9. Arch Ophthalmol 114:286–292, 1996 214. McCuen BW et al: Vitrectomy with silicone oil or perfluoropropane gas in eyes with severe
proliferative vitreoretinopathy. Silicone Study Report 3. Retina 13:279–284, 1993 215. Group SS: Vitrectomy with silicone oil or perfluoropropane gas in eyes with severe
proliferative vitreoretinopathy: Results of a randomized clinical trial. Silicone
Study Report 2. Arch Ophthalmol 110:780–792, 1992 216. Group SS: Vitrectomy with silicone oil or sulfur hexafluoride gas in eyes with severe
proliferative vitreoretinopathy: Results of a randomized clinical
trial. Silicone Study Report 1. Arch Ophthalmol 110:770–779, 1992 217. Abrams GW et al: Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative
vitreoretinopathy: Results of additional and long-term follow-up. Silicone
Study Report 11. Arch Ophthalmol 115:335–344, 1997 218. Lucke KH, Foerster MH, Laqua H: Long-term results of vitrectomy and silicone oil in 500 cases of complicated
retinal detachments. Am J Ophthalmol 104:624–633, 1987 219. Sell CH et al: Long-term results of successful vitrectomy with silicone oil for advanced
proliferative vitreoretinopathy. Am J Ophthalmol 103:24–28, 1987 220. Campochiaro PA: A small piece of the PVR puzzle is put into place. Silicone Study. Arch Ophthalmol 115:407–408, 1997 221. Abrams GW et al: The incidence of corneal abnormalities in the Silicone Study. Silicone
Study Report 7. Arch Ophthalmol 113:764–769, 1995 222. Kampik A, Gandorfer A: Silicone oil removal strategies. Semin Ophthalmol 15:88–91, 2000 223. Jonas JB, Budde WM, Knorr HL: Timing of retinal redetachment after removal of intraocular silicone oil
tamponade. Am J Ophthalmol 128:628–631, 1999 224. Coll G et al: Perfluorocarbon liquid in the management of retinal detachment with proliferative
vitreoretinopathy. Ophthalmology 102:630–639, 1995 225. Wiedemann P et al: Adjunctive daunorubicin in the treatment of proliferative vitreoretinopathy: Results
of a multicenter clinical trial. Daunomycin Study Group. Am J Ophthalmol 126:550–559, 1998 226. Yang CS et al: An intravitreal sustained release triamincinolone and 5-fluorouracil codrug
in treatment of experimental proliferative vitreoretinopathy. Arch Ophthalmol 116:69–77, 1998 227. Chang S et al: Giant retinal tears: Surgical techniques and results using perfluorocarbon
liquids. Arch Ophthalmol 107:761–766, 1989 228. Kreiger AE, Lewis H: Management of giant retinal tears without scleral buckling: Use of radical
dissection of the vitreous base and perfluoro-octane and intraocular
tamponade. Ophthalmology 99:491–497, 1992 229. Ie D et al: The use of perfluoro-octane in the management of giant retinal tears without
proliferative vitreoretinopathy. Retina 14:323–328, 1994 230. Glaser BM et al: Perfluoro-octane in the treatment of giant retinal tears with proliferative
vitreoretinopathy. Ophthalmology 98:1613–1621, 1991 231. Chang S et al: Perfluorocarbon liquids in the management of traumatic retinal detachments. Ophthalmology 96:785–791, 1989 232. Gass JD: Reappraisal of biomicroscopic classification of stages of development of
a macular hole. Am J Ophthalmol 119:752–759, 1995 233. de Bustros S: Vitrectomy for prevention of macular holes: Results of a
randomized multicenter clinical trial. Vitrectomy for Prevention of Macular
Hole Study Group. Ophthalmology 101:1055–1059, 1994; discussion, 1060 234. Kim JW et al: Prospective randomized trial of vitrectomy or observation for stage 2 macular
holes. Vitrectomy for Macular Hole Study Group. Am J Ophthalmol 121:605–614, 1996 235. Kelly NE, Wendel RT: Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol 109:654–659, 1991 236. Thompson JT et al: Comparison of recombinant transforming growth factor-beta-2 and placebo
as an adjunctive agent for macular hole surgery. Ophthalmology 105:700–706, 1998 237. Freeman WR et al: Vitrectomy for the treatment of full-thickness stage 3 or 4 macular
holes: Results of a multicentered randomized clinical trial. The
Vitrectomy for Treatment of Macular Hole Study Group. Arch Ophthalmol 115:11–21, 1997 (published erratum appears in Arch Ophthalmol 115:636, May 1997) 238. Smiddy WE, Pimentel S, Williams GA: Macular hole surgery without using adjunctive additives. Ophthalmic Surg Lasers 28:713–717, 1997 239. Wendel RT et al: Vitreous surgery for macular holes. Ophthalmology 100:1671–1676, 1993 240. Park DW et al: Macular hole surgery with internal-limiting membrane peeling and intravenous
air. Ophthalmology 106:1392–1398, 1999 241. Spraul C, Grossniklaus H: Vitreous hemorrhage. Surv Ophthalmol 42:3–39, 1997 242. Smiddy WE et al: Vitrectomy for nondiabetic vitreous hemorrhage: Retinal and choroidal vascular
disorders. Retina 8:88–95, 1988 243. Isernhagen RD et al: Vitrectomy for nondiabetic vitreous hemorrhage: Not associated with vascular
disease. Retina 8:81–87, 1988 244. Pournaras CJ et al: Macular epiretinal membranes. Semin Ophthalmol 15:100–107, 2000 245. Poliner LS et al: Surgical management of premacular fibroplasia. Arch Ophthalmol 106:761–764, 1988 246. de Bustros S et al: Vitrectomy for idiopathic epiretinal membranes causing macular pucker. Br J Ophthalmol 72:692–695, 1988 247. Cherfan GM et al: Nuclear sclerotic cataract after vitrectomy for idiopathic epiretinal membranes
causing macular pucker. Am J Ophthalmol 111:434–438, 1991 248. de Bustros S et al: Nuclear sclerosis after vitrectomy for idiopathic epiretinal membranes. Am J Ophthalmol 105:160–164, 1988 249. Williams D et al: Results and prognostic factors in penetrating ocular injuries with retained
intraocular foreign bodies. Ophthalmology 95:911–916, 1988 250. DeJuan E, Sternberg P Jr, Michels R: Penetrating ocular injuries: Types of injuries and visual results. Ophthalmology 90:1318–1322, 1983 251. Greven CM et al: Intraocular foreign bodies. Management, prognostic factors and visual outcomes. Ophthalmology 107:608–612, 2000 252. Brown G, Tasman W, Benson W: BB-gun injuries to the eye. Ophthalmic Surg 16:505–508, 1985 253. Cleary PE, Ryan SJ: Vitrectomy in penetrating eye injury. Results of a controlled trial of
vitrectomy in an experimental posterior penetrating injury in the rhesus
monkey. Arch Ophthalmol 106:287–292, 1981 254. Abrams GW, Topping TM, Machemer R: Vitrectomy for injury: The effect on intraocular proliferation following
perforation of the posterior segment of the rabbit eye. Arch Ophthalmol 97:743–750, 1979 255. Endophthalmitis Vitrectomy Study Group: Results of the Endophthalmitis
Vitrectomy Study: A randomized trial of immediate vitrectomy and of intravenous
antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch
Ophthalmol 113:1479–1496, 1995 256. Clark WL et al: Treatment strategies and visual acuity outcomes in chronic postoperative Propionibacterium acnes endophthalmitis. Ophthalmology 106:1665–1670, 1999 257. Aldave A et al: Treatment strategies for postoperative Propionibacterium acnes endophthalmitis. Ophthalmology 106:2395–2401, 1999 258. Smiddy WE: Treatment outcomes of endogenous fungal endophthalmitis. Curr Opin Ophthalmol 9:66–70, 1998 259. Essman TF et al: Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers 28:185–194, 1997 260. Mieler W et al: Retained intraocular foreign bodies and endophthalmitis. Ophthalmology 97:1532–1538, 1990 261. Diamond J, Kaplan HJ: Uveitis: Effect of vitrectomycombined with lensectomy. Ophthalmology 86:1320–1327,1979 262. Foster CS, Fong L, Singh G: Cataract surgery and intraocular lens implantation in patients with uveitis. Ophthalmology 96:281–288, 1989 263. Michelson J, Friedlaender M, Nozik R: Lens implantsurgery in pars planitis. Ophthalmology 97:1023–1026,1990 264. Belmont J et al: Vitrectomy in ocular toxocariasis. Arch Ophthalmol 100:1912–1915, 1982 265. Jabs D et al: Retinal detachments in patients with cytomegalovirus retinitis. Arch Ophthalmol 109:794–799, 1991 266. Clarkson J et al: Retinal detachment following the acute retinal necrosis syndrome. Ophthalmology 91:1665–1668, 1984 267. Fisher J et al: The acute retinal necrosis syndrome. Ophthalmology 89:1309–1316, 1982 268. Freeman HM et al: Surgical repair of rhegmatogenousretinal detachment in immunosuppressed
patients withcytomegalovirus retinitis. Ophthalmology 99:466–474,1992 269. Blumenkranz M et al: Visual results and complications after retinal after retinal reattachment
in the ARN syndrome: The influence of operative technique. Retina 9:170–174, 1989 270. Fung WE: Vitrectomy for chronic aphakic cystoid macular edema: Results of a national, collaborative, prospective, randomized investigation. Ophthalmology 92:1102–1111, 1985 271. Harbour JW et al: Pars plana vitrectomy for chronic pseudophakic cystoid macular edema. Am J Ophthalmol 120:302–307, 1995 272. Pendergast SD et al: Vitrectomy for chronic pseudophakic cystoid macular edema. Am J Ophthalmol 128:317–323, 1999 273. Hikichi T, Yoshida A, Trempe C: Course of vitreomacular traction syndrome. Am J Ophthalmol 119:55–61, 1995 274. Smiddy WE et al: Vitrectomy for macular traction caused by incomplete vitreous separation. Arch Ophthalmol 106:624–628, 1988 275. Lakhanpal V et al: A new modified vitreoretinal approach in the management of massive suprachoroidal
hemorrhage. Ophthalmology 96:793–800, 1989 276. Hochman MA et al: Subretinal hemorrhage. Surv Ophthalmol 42:197–213, 1997 277. Koshibu A: Ultrastructural studies on absorption of experimentally produced subretinal
hemorrhage. III. Absorption of erythrocyte breakdown products and
retinal hemosiderosis at the last stage. Nippon Ganka Gakkai Zasshi 83:386–400, 1979 278. Toth CA et al: Fibrins directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol 109:723–729, 1991 279. Glatt H, Machemer R: Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol 94:762–773, 1982 280. Berrocal M, Lewis M, Flynn HW: Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol 122:486–493, 1996 281. Shiraga F et al: Surgical treatment of submacular hemorrhage associated with idiopathic
polypoidal choroidal vasculopathy. Ophthalmology 128:147–154, 1999 282. The International Committee for the Classification of the Late Stages of
Retinopathy of Prematurity: An international classification of retinopathy
of prematurity. II. The classification of retinal detachment. Arch
Ophthalmol 105:906–912, 1987 283. Maguire AM, Trese MT: Lens-sparing vitreoretinal surgery in infants. Arch Ophthalmol 110:284–286, 1992 284. Trese MT: Management of early ROP detachments: Lens sparing vitrectomy. Presented
at Retina 2000: Management of Posterior Segment Disease. Dallas, 2000 285. Hirose T et al: Vision in stage 5 retinopathy of prematurity after retinal reattachment
by open-sky vitrectomy. Arch Ophthalmol 111:345–349, 1993 286. Fuchino Y et al: Long-term follow up of visual acuity in eyes with stage 5 retinopathy of
prematurity after closed vitrectomy. Am J Ophthalmol 120:308–316, 1995 287. Trese MT, Droste P: Long-term postoperative results of a consecutive series
of stages 4 and 5 retinopathy of prematurity. Ophthalmology105:992–997, 1998 288. Macular Photocoagulation Study Group: Laser photocoagulation of subfoveal
neovascular lesions in age-related macular degeneration: Updated findings
from two clinical trials. Arch Ophthalmol 111:1200–1209, 1993 289. Photodynamic Therapy Study Group: Photodynamictherapy of subfoveal choroidal
neovascularization in age-related macular degeneration: One year
results of two randomized clinical trials. TAP Report 1. Treatment of
age-related macular degeneration with photodynamic therapy (TAP) study
group. Arch Ophthalmol 117:1329–1345,1999 290. Merrill P et al: Surgical removal of subfoveal choroidal neovascularization in age-related
macular degeneration. Ophthalmology 106:782–789, 1999 291. Lewis H, Vanderburg Medendorp S: Tissue plasminogen activator-assisted surgical excision of subfoveal choroidal
neovascularization in age-related macular degeneration: A randomized, double-masked
trial. Ophthalmology 104:1847–1852, 1997 292. Thomas MA et al: Visual results after surgical removal of subfoveal choroidal neovascular
membranes. Ophthalmology 101:1384–1396, 1994 293. Holekamp NM et al: Surgical removal of subfoveal choroidal neovascularization in presumed
ocular histoplasmosis: Stability of early visual results. Ophthalmology 104:22–26, 1997 294. Lambert HM: The management of subfoveal choroidal neovascular membranes and hemorrhage. Semin Ophthalmol 15:92–99, 2000 295. Vander JF: Macular translocation. Curr Opin Ophthalmol 11:159–165, 2000 296. DeJuan E, Fujii G: Further experience with limited macular translocation. Presented
at Retina 2000: Management of posterior segment disease. Dallas, 2000 297. Lewis H et al: Macular translocation for subfoveal choroidal neovascularization in age-related
macular degeneration. A prospective study. Am J Ophthalmol 128:135–146, 1999 298. Haller JA et al: Limited macular translocation for neovascular maculopathy. Semin Ophthalmol 15:81–88, 2000 299. Eckardt C: Further experience with 360-degree retinal rotation. Presented
at Retina 2000: Management of posterior segment disease. Dallas, 2000 300. Schwartz E: Corneal sensitivity in diabetics. Arch Ophthalmol 91:174–178, 1974 301. Ohashi Y et al: Aldose reductase inhibitor (CT-112) eyedrops for diabetic corneal epithelialopathy. Am J Ophthalmol 105:233–238, 1988 302. Foulks G et al: Factors related to corneal epithelial complications after closed vitrectomy
in diabetics. Arch Ophthalmol 97:1076–1078, 1979 303. Edelhauser HF et al: The effect of phenylephrine on the cornea. Arch Ophthalmol 97:937–947, 1979 304. Carter JB et al: Iatrogenic retinal breaks complicating pars plana vitrectomy. Ophthalmology 97:848–854, 1990 305. Sjaarda RN et al: Distribution of iatrogenic retinal breaksin macular hole surgery. Ophthalmology 102:1387–1392,1995 306. Stern W: Complications of vitrectomy. Semin Ophthalmol 15:205–212, 2000 307. Oyakawa RT et al: Complications of vitreous surgery for diabetic retinopathy. I. Intraoperative
complications. Ophthalmology 90:517–521, 1983 308. Piper JG et al: Perioperative choroidal hemorrhage at pars plana vitrectomy: A case-control
study. Ophthalmology 100:699–704, 1993 309. Tabandeh H et al: Suprachoroidal hemorrhage during pars plana vitrectomy: Risk factors and
outcomes. Ophthalmology 106:236–242, 1999 310. McDonald HR, Harris MJ: Operating microscope-induced retinal phototoxicity during pars plana vitrectomy. Arch Ophthalmol 106:521–523, 1988 311. Michels M et al: Macular phototoxicity caused by fiberoptic endoillumination during pars
plana vitrectomy. Am J Ophthalmol 114:287–296, 1992 312. Postel E et al: Long-term follow-up of iatrogenic phototoxicity. Arch Ophthalmol 116:753–757, 1998 313. Tolentino FI, Freeman HM, Tolentino FL: Closed vitrectomy in the management of diabetic traction retinal detachment. Ophthalmology 87:1078–1089, 1980 314. Chung H et al: Reevaluation of corneal complicationsafter closed vitrectomy. Arch Ophthalmol 106:916–919,1988 315. Schachat AP et al: Complications of vitreous surgery from diabetic retinopathy. II. Post-operative
complications. Ophthalmology 90:522–530, 1983 316. Helbig H et al: Rubeosis iridis after vitrectomy for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 236:730–733, 1998 317. Blankenship G: Pre-operative iris rubeosis and diabetic vitrectomy results. Ophthalmology 87:176–182, 1980 317a. Stefanssen E, Landers MB III, Wolbarsyat ML: Increased retinal oxygen supply following panretinal photocoagulation and
vitrectomy and lensectomy. Trans Am Ophthalmol Soc 79:307, 1981 318. Blankenship G: The lens influence on diabetic vitrectomy results. Arch Ophthalmol 98:2196–2198, 1980 319. Lewis H, Abrams GW, Williams GA: Anterior hyaloidal fibrovascular proliferation after diabetic vitrectomy. Am J Ophthalmol 104:607–613, 1987 320. Campbell D et al: Glaucoma occurring after closed vitrectomy. Am J Ophthalmol 83:63–69, 1977 321. Leaver P, Grey R, Garner A: Silicone oil injection in the treatment of massive preretinal traction. II. Late
complications in 93 eyes. Br J Ophthalmol 63:361–367, 1979 322. Ando F: Intraocular hypertension resulting from pupillary block by silicone oil. Am J Ophthalmol 99:87–88, 1985 323. Chang S, Sparrow JR: Vitreous substitutes. In Guyer D et al (eds): Retina, Vitreous, Macula. Philadelphia: WB Saunders, 1999:1328 324. Desai UR et al: Intraocular pressure elevation after simple pars plana vitrectomy. Ophthalmology 104:781–786,1997 325. Park SS et al: Posterior segment complications after vitrectomy for macular hole. Ophthalmology 102:775–781,1995 326. Thompson JT et al: Progression of nuclear sclerosis and long-term visual results of vitrectomy
with transforming growth factor beta-2 for macular holes. Am J Ophthalmol 119:48–54, 1995 327. Krzystolik M, D'Amico DJ: Complications of intraocular tamponade: Silicone oil versus intraocular
gas. Int Ophthalmol Clin 40:197, 2000 328. Novak MA et al: Vitreous hemorrhage after vitrectomyfor diabetic retinopathy. Ophthalmology 91:1485–1489,1984 329. Blankenship GW: Management of vitreous cavity hemorrhage following pars plana vitrectomy
for diabetic retinopathy. Ophthalmology 93:39–44, 1986 330. Margherio RR, Cox MS, Trese MT: Removal of epimacular membranes. Ophthalmology 92:1075–1083, 1985 331. Michels RG: Vitrectomy for macular pucker. Ophthalmology 91:1384–1388, 1984 332. McDonald HR, Verre W, Aaberg TM: Surgical management of idiopathic epiretinal membranes. Ophthalmology 93:978–983, 1986 333. Banker AS et al: Vision-threatening complications of surgery for full-thickness
macular holes. Vitrectomy for Macular Hole Study Group. Ophthalmology 104:1442–1452, 1997; discussion, 1452–1453 334. Buettner H, Machemer R: Histopathologic findings in human eyes after pars plana vitrectomy and
lensectomy. Arch Ophthalmol 95:2029–2033, 1977 335. Cohen SM et al: Endophthalmitis after pars plana vitrectomy. The Postvitrectomy Endophthalmitis
Study Group. Ophthalmology 102:705–712, 1995 336. Gass JD: Sympathetic ophthalmia following vitrectomy. Am J Ophthalmol 93:552–558, 1982 337. Croxalto J et al: Sympathetic ophthalmia after pars plana vitrectomy-lensectomy for endogenous
bacterial endophthalmitis. Am J Ophthalmol 91:342–346, 1981 338. Gasch A et al: Postoperative sympathetic ophthalmia. Int Ophthalmol Clin 69–84, 2000 |