SIGNIFICANCE
The most common cause for ultimate failure after retinal reattachment surgery is the development of PVR.62–65 Current techniques of retinal detachment repair, including scleral buckling and pneumatic retinopexy, are successful in about 90% of cases, but 10% still ultimately fail because of the development of PVR. To manage these patients successfully, one must understand the pathology, pathobiology, and pathophysiology of the disease along with its natural biologic behavior. Successful treatment requires a complete understanding of the anatomic changes as the disease progresses, the intended goals of treatment, and the treatment alternatives.66–70
PATHOLOGY
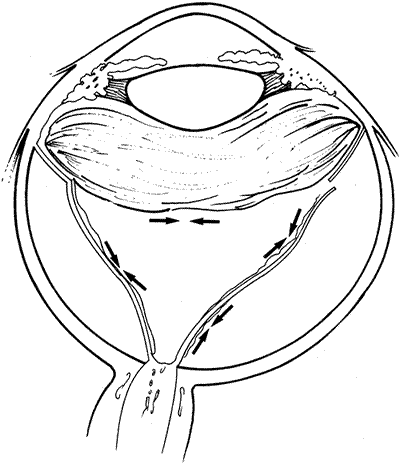
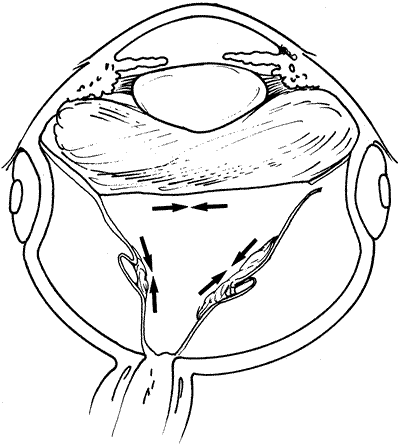
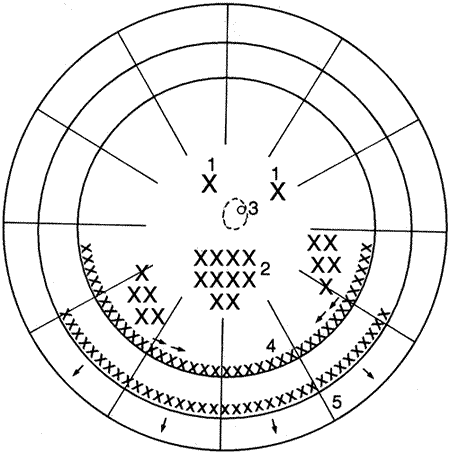
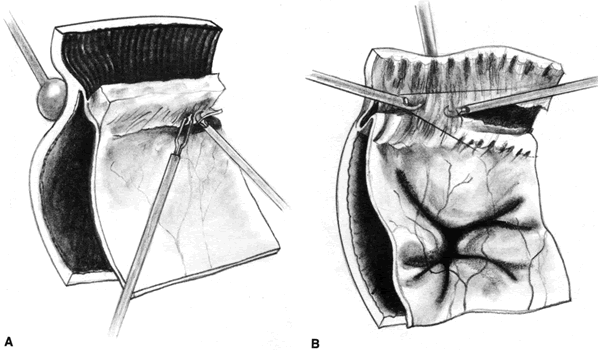
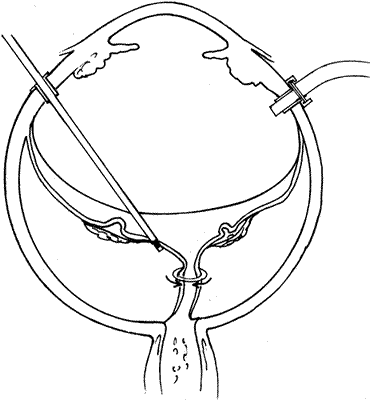
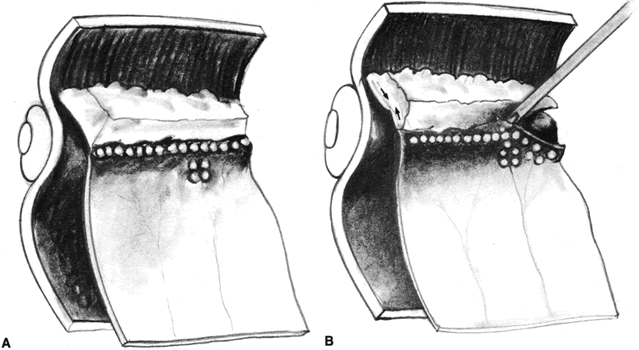
PVR is the pathologic growth of cells that are capable of forming cellular and collagenous membranes on the anterior and posterior surfaces of the retina, in the vitreous cavity (particularly the posterior hyaloid face), and in the vitreous base (Figs. 1 and 2).2,71–75 Predisposing factors that contribute to the development of PVR include chronic retinal detachments (>1 month), aphakia, vitreous hemorrhage, and increasing size of retinal breaks. In particular, patients with giant retinal tears (tear greater than 3 clock-hours in size) with a folded-over posterior flap are at high risk for PVR.76
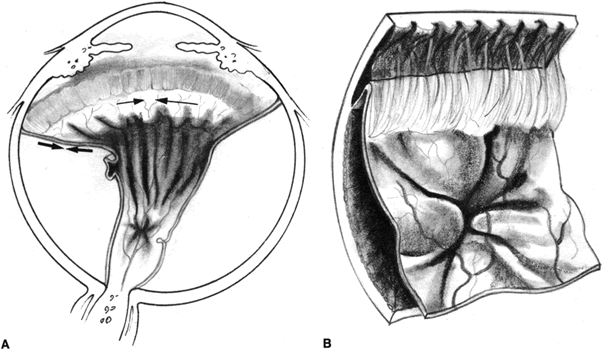
The proliferative process can also occur anteriorly with growth of cellular membranes over the pars plicata of the ciliary body, the posterior surface of the iris, and even the pupillary margin.77,78 Cells contained within these membranes have contractile properties that can result in traction on either side of the retina, the ciliary body, and the posterior iris surface. Epiretinal proliferations may occur focally, giving rise to full-thickness radiating retinal folds in the pattern of a star or diffusely giving rise to large areas of fixed, irregular retinal folds.63 Subretinal proliferations usually begin as membranous sheets that are not clinically visible. With contraction, the membranes develop numerous holes that may give a moth-eaten appearance to the posterior retinal surface. As the holes enlarge the membranes roll up, forming strands or bands that may be white or pigmented.79–81 Proliferation and contraction of membranes at the junction of the detached hyaloid face and peripheral retina (usually equatorial in location) results in central displacement of the retina.82 The contraction forces result in radial retinal folds with a funnel-shaped retinal detachment posterior to and stretching of the retina anterior to the junction zone (Fig. 3A). Lastly, anterior proliferation, which frequently but not exclusively occurs in the remnants of the vitreous base after vitreous surgery, can result in dragging of the peripheral retina and posterior vitreous base toward the membranes' anterior attachments (pars plicata, posterior iris surface, pupillary margin). The resultant pathoanatomy of the contraction is a circumferential peripheral trough of varying width and depth (see Fig. 3B).77,82
It is commonly believed that the primary effector cell for the development of PVR is the retinal pigment epithelial (RPE) cell.2,73,82 Ultrastructural analysis of epiretinal and subretinal membranes removed from human eyes with PVR and animal eyes with experimentally induced PVR have shown a predominance of RPE cells.71,74,78–81,83 Additional cell types identified in these specimens include glial cells (fibrous astrocytes), macrophages, fibrocytes, and myofibroblast-like cells. These latter cells usually have characteristics of fibrocytes, but occasionally, they contain structures more commonly seen in RPE or glial cells.72,73,75,77–80 The exact origin of these cells is controversial,84 but they probably represent RPE and glial cells that are capable of transforming into myofibroblast-like cells with the capacity to synthesize collagen and contract.2,72,85,86
A frequent finding in epiretinal membrane formation is the deposition of pigmented dots or clumps on the inner retinal surface. They tend to be irregularly distributed, but due to gravity, they are most concentrated over the inferior retina.87 Laqua and Machemer73 have shown histologically that these dots are composed of pigmented epithelial macrophages. Their contributory role to the actual production of collagenous epiretinal membranes is believed to be negligible.
The composition of subretinal membranes seems to depend somewhat on the age of the detachment. The deposition of RPE cells on the posterior retinal surface can occur within 1 week of a detachment.2,88 The appearance of subretinal white precipitates (presumably pigment epithelial macrophages) are the earliest ophthalmoscopic sign of subretinal membrane formation.73,88 They tend to be irregularly distributed, but the heaviest aggregates are usually seen on the inferior retina. The growth pattern of these membranes is diffuse at first, but with cellular contraction, weak areas break, holes result, and subretinal strands are formed. The pigmentation of the subretinal bands depends on the concentration of pigment in the cells that make up the proliferation. As collagen is produced, the membranes become more opaque and take on a yellow-white hue. RPE cells are the predominant component of the subretinal proliferations. In contrast, glial cells are rarely seen. The one exception occurs in chronically detached, gliotic appearing retinas where extensive subretinal glial membranes are found in association with a dramatic loss of photoreceptors. The membranes are typically invisible on ophthalmoscopy but may give the retina a moth-eaten appearance.2,71–73,79,88
PATHOBIOLOGY
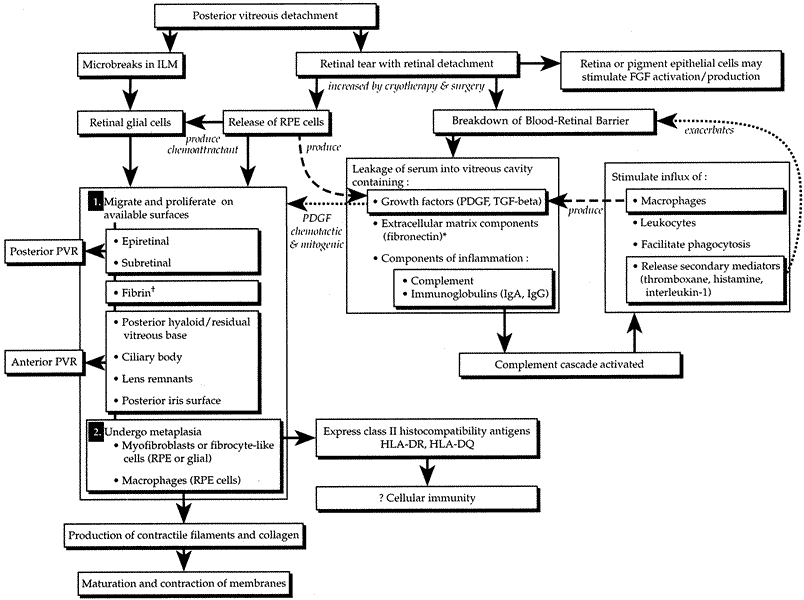
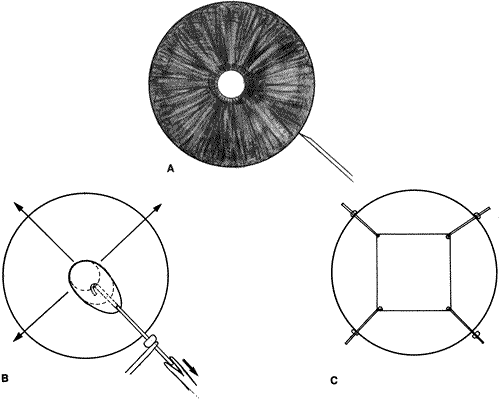
The pathobiology of PVR is not yet fully understood, although research continues to shed new light on its basic mechanisms.2,71,73 When a retinal tear occurs, RPE cells are released through the break into the vitreous cavity (Fig. 4). As the retina detaches, some RPE may remain adherent to the retinal flap. With time, these areas fall off and seed the vitreous cavity. In addition, there is in vivo89 and in vitro90 experimental evidence to suggest that transscleral cryotherapy to the retinal breaks disperses large numbers of viable RPE cells into the vitreous cavity.91 The dispersed or stimulated RPE cells have been shown to be capable of releasing chemoattractants, inducing migration and chemotaxis. Experimentally, human RPE cells have been shown to release a chemoattractant for retinal astrocytes.92 They also produce and release platelet-derived growth factor (PDGF), which is a known mitogen and chemoattractant for human RPE and glial cells.93,94 Studies have also demonstrated that RPE cells are a source of transforming growth factor-β (TGF-β), which stimulates fibroblasts to proliferate and synthesize collagen and fibronectin.95 TGF-β is also a known chemoattractant for monocytes.96,97
The breakdown of the blood–retina barrier in patients with retinal detachments is demonstrated by flare in the vitreous cavity. This breakdown is exacerbated by cryotherapy and/or surgical intervention. Disruption of the blood–retina barrier permits serum with its various growth factors (PDGF, TGF-β) and components of inflammation (e.g., complement, immunoglobulins [IgG, IgA, IgE]) and extracellular matrix (e.g., fibronectin) to gain access to the vitreous cavity.98 Serum itself has been shown to be a potent stimulator of RPE cell migration and is chemotactic for human RPE99 and retinal derived glial cells.100 The primary factor in serum responsible for RPE migration and chemotaxis has been shown in vitro to be fibronectin.99 Fibronectin is an integral component of extracellular matrix and has been found to be a potent stimulator of cellular migration and proliferation for a variety of cells.101 Interestingly, in vivo studies looking at vitrectomy specimens from patients with PVR have shown levels of fibronectin seven times higher than those in eyes with long-standing macular puckers or uncomplicated retinal detachments.102 A secondary but still potent stimulator of RPE migration found in serum is PDGF (see Fig. 4).93
The role of inflammation in the pathogenesis of PVR remains to be defined. Studies, however, have identified various complement components (C1q, C4, C3, C3c, C3d) in specimens obtained from patients undergoing surgery for PVR, suggesting that the complement system is activated in patients with PVR.103–105 Some macrophages and B or T8 lymphocytes have also been identified.105 These findings, when taken together, suggest the possibility of an autoimmune response. The expression by various cells such as RPE, nonpigmented ciliary epithelium, and astrocytes of class II histocompatibility antigens (HLA-DR, HLA-DQ) also lends support to an autoimmune reaction with complement activation and possible cell-mediated immunity (see Fig. 4).104–106
PATHOPHYSIOLOGY
Among the normal functions of the retinal pigment epithelium are the renewal of photoreceptor outer segments and the maintenance of a deturgesced subretinal space. When a retinal tear occurs, RPE cells normally adherent to Bruch's membrane disperse or migrate into the vitreous cavity (predominantly inferiorly due to gravity),87,107 as well as onto the epiretinal and subretinal surfaces of the retina. The separation of RPE from Bruch's membrane may be due, in part, to proteolytic enzymes and proteins with counter-adhesive properties.108 The dispersion of viable RPE cells is the inciting event in the pathogenesis of PVR (see Fig. 4) and is increased when cryotherapy, which weakens the adherence between the RPE and its basement membrane, is followed by scleral depression.90,91 In addition, the blood–retina barrier is further disrupted.98 Serum with inflammatory mediators, growth factors, and extracellular matrix components enters the vitreous cavity. Freed viable RPE cells are exposed to a different extracellular milieu that stimulates them to proliferate and undergo a morphologic change from an epithelial-like appearance to one resembling a fibroblast or mesenchymal cell.109 The RPE cells release substances that are capable of attracting other cells such as astrocytes,92 fibroblasts,110 and monocytes.96 These cells are stimulated to proliferate by intraocular or plasma-derived growth factors.95,111 Some of these same cells are capable of producing growth factors, extracellular matrix (fibronectin), and collagen.91,112 Fibronectin and growth factors increase in the vitreous cavity and attract more pigment epithelial and fibroblast-like cells.95,99,102 More collagen is produced, cells contract, collagen matures, and retinal traction results. Thus, a potential PVR life cycle is set up.
CLASSIFICATION SYSTEMS
Early Versions
The classification system for PVR (massive vitreous retraction [MVR], massive preretinal retraction [MPR], massive periretinal proliferation [MPP]) has undergone several revisions since its early description.2,63,113–115 The classification systems were developed to describe the anatomic appearance of epiretinal and subretinal membranous proliferations. In 1983, the Retina Society developed a grading system of PVR that provided a more detailed description of the various stages of the disease.63 The stages were divided into four subgroups:
Grade A consists of pigmented clumps (RPE cells) in the vitreous
cavity and on the inferior retinal surface. Flare is present in the
vitreous cavity, and the posterior vitreous face is less mobile.
Grade B is defined as wrinkling of the inner retinal surface and retinal
breaks with rolled edges. The retina and vitreous are also less mobile.
Grade C is subdivided according to the number of quadrants involved with
star folds. C-1 indicates a fixed fold in one quadrant; C-2 and
C-3, fixed folds in two and three quadrants, respectively.
Grade D is defined as fixed folds in all four quadrants. The classification
is subdivided based on the funnel appearance of the retinal detachment
when viewed through a 20-diopter indirect lens. A wide-open
funnel is D-1, a narrow funnel where equatorial retinal
is visible in the 20-diopter field but the optic disc is visible
is D-2. D-3 is reserved for narrow funnel retinal detachments
with obscuration of the disc.
Revised System
The major drawbacks of the original Retina Society classification system were that it primarily described the effects of membranes on postequatorial retina and that it failed to reliably correlate with the anatomic and visual outcomes. In addition, it did not address anterior proliferations, which frequently occur in patients who have undergone vitreoretinal surgery and which are the primary cause of failure of vitreoretinal reattachment procedures. In 1989, as part of a multicenter, prospective, randomized, clinical trial examining the efficacy of extended gas versus silicone oil tamponade, a new classification system was devised to more accurately describe the types of contraction found in this disease.116 Subsequently, in 1991, a committee appointed by the Retina Society, using the Silicone Study information and input from other investigators published a revised classification system.82 The new classification retains grades A and B but modifies C and eliminates D.
Grade C, as in the old classification, is defined by full-thickness retinal folds. It is subdivided, however, into anterior and posterior (equator is dividing line), number of clock-hours involved, and type of proliferation (focal, diffuse, subretinal, circumferential, and anterior displacement) (Table 1) (Fig. 5). Anterior displacement of the vitreous base tends to occur in eyes that have undergone previous vitreoretinal surgery. It can, however, occasionally occur in long-standing untreated PVR. The major changes in the grading system from the original classification are the distinction between anterior and posterior proliferation, contraction types, and the use of clock-hours to describe the extent of the abnormalities.
Table 1. Revised Grading System for Proliferative Vitreoretinopathy
| Grade | Location* | Extent† | Type of Contraction | Features |
| A | Vitreous flare; pigment clumps in vitreous and on retina | |||
| B | Inner retinal wrinkling; retinal stiffness; vessel tortuosity; rolled and irregular edges of retinal break(s): decreased vitreous mobility | |||
| C | Posterior (P) | 1–12 | Focal(1) | Star folds |
| Posterior | 1–12 | Diffuse (2) | Confluent star folds. Optic disc may not be visualized | |
| Posterior/anterior | 1–12 | Subretinal (3) | Annular strand near disc; pigmented or nonpigmented linear strands; moth-eaten-appearing sheets | |
| Anterior (A) | 1–12 | Circumferential (4) | Contraction along posterior margin of vitreous base with central displacement of the retina; peripheral retina stretched; posterior retina in radial folds | |
| Anterior | 1–12 | Anterior displacement (5) | Vitreous base pulled anteriorly; peripheral retinal trough of varying width: ± stretching of ciliary processes; ± obscuration of ciliary processes by membranes; ± iris retraction |
*Location relative to the equator.
† Extent of involvement in clock hours.
(Data from Machemer R, Aaberg TM, Freeman HM et al: An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol 112: 159, 1991)
SURGICAL MANAGEMENT
Surgical Objectives
The surgical techniques used to manage patients with a rhegmatogenous retinal detachment complicated by PVR vary dramatically from those used to repair “routine” rhegmatogenous retinal detachments. The primary goal of vitreous surgery is to convert a retinal detachment complicated by severe PVR into a “routine” rhegmatogenous retinal detachment and then to repair the “routine” retinal detachment. What follows is a description of some of the various vitreous surgery techniques used to accomplish these goals.
Preoperative Evaluation
It is during the preoperative examination that the surgeon formulates the surgical approach. A complete ophthalmologic examination including best-corrected visual acuity, intraocular pressures, slit-lamp biomicroscopy, gonioscopy, and funduscopy is performed. During gonioscopy the presence of any anterior pathology such as rubeosis, peripheral anterior synechiae, or a cyclodialysis cleft is noted. Funduscopy should include indirect ophthalmoscopy and slit-lamp retinal biomicroscopy to help delineate vitreoretinal anatomy. Indirect ophthalmoscopy should be performed in conjunction with scleral depression. All findings need to be clearly annotated and the PVR stage classified according to the new Retina Society classification scheme.82
Preoperative counseling of these patients should include a full discussion of the guarded anatomic and visual results in patients undergoing PVR surgery and the frequent necessity for reoperation. In addition, the risks and complications of the procedure should be carefully explained, including infection, increased or decreased intraocular pressure, recurrent retinal detachment, recurrent proliferation, hemorrhage, phthisis, keratopathy, and if phakic, cataract formation.
Anesthesia
Whether to use retrobulbar or general anesthesia or a combination of the two is generally decided by the surgeon based on his or her preference, the patient's preference, the patient's general medical condition, and the specific procedure planned. Clipping of the patient's eyelashes is optional but should be done before prepping the eye. Once the eye is prepped and draped, a lid speculum that will produce a wide palpebral fissure is inserted. If a narrow palpebral fissure restricts opening of the lid speculum, a lateral canthotomy may be performed to provide an adequate space for rotation of the globe during surgery.
Sclerotomies
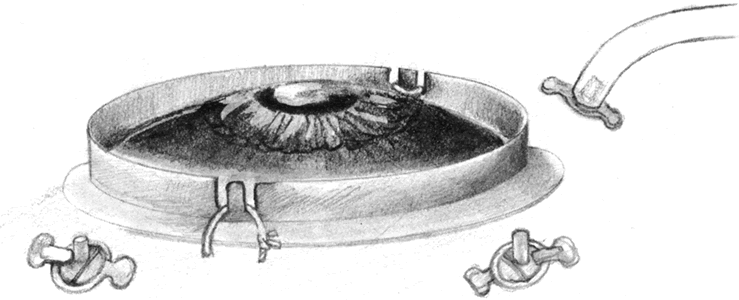
If a buckle has been previously placed and has been judged to be adequate preoperatively, then conjunctival peritomies may be performed in preparation for the sclerotomies. The placement of the conjunctival peritomies is dependent on the intraocular pathology and status of the sclera. Typically they are placed supranasally and temporally. If a scleral buckle has not been previously placed or revision of the scleral buckle is planned, a 360-degree conjunctival peritomy should be performed and the rectus muscles isolated with sutures. Bleeding is controlled using cautery, but care is taken to prevent excessive treatment. Sclerotomies are performed in the inferotemporal (infusion port), supertemporal, and supranasal quadrants, 3 to 4 mm posterior to the limbus depending on whether the eye is phakic. Sclerotomies can be performed more anteriorly in eyes where anterior displacement has resulted in iridoretinal adhesions. If the sclera is too macerated in the region of previous sclerotomies, a radial sclerotomy may be performed. Metal cannulas that permit the exchange of 19- or 20-gauge instruments through the sclera are used by some surgeons to prevent maceration of the sclera and to protect the vitreous base. Many surgeons incorporate a lens ring secured to the eye so that various contact lenses (direct viewing or widefield) may be placed to facilitate membrane dissection and other procedures (Fig. 6). Alternatively, some surgeons prefer to use a noncontact widefield viewing system. These viewing systems have largely replaced the hand-held infusion contact lens system which has been shown to produce more keratopathy.117
Lensectomy
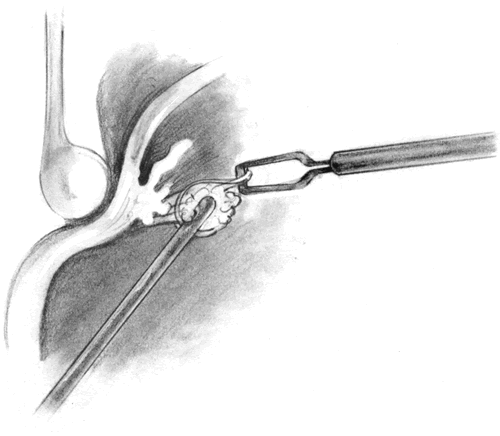
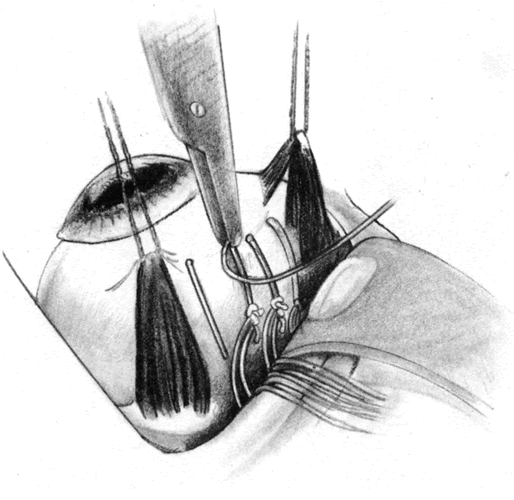
The role of routine lensectomy in PVR surgery is changing. In cases of anterior PVR, a lensectomy is performed to allow extreme anterior dissection and to accommodate an extended intraocular tamponade. This is usually performed through the superior pars plana sclerotomies with a phacofragmentor (Fig. 7). Once the nuclear material is removed, the residual cortical material and posterior lens capsule are removed using a microcutter. Finally, a hole is made in the anterior lens capsule and all capsular remnants are removed. A microforceps can be used to grasp the cut edge of the capsule and place it on traction so that the zonulae may be severed with the microcutter. This can be done in conjunction with scleral indentation (Fig. 8). It is helpful to remove all capsular and equatorial lens cellular remnants because they can act as scaffolds for future proliferation. In cases with primarily posterior PVR, however, routine lensectomy can frequently be avoided and subsequent postoperative inflammation reduced.
|
Pseudophakos
The management of anterior chamber and posterior chamber intraocular lenses is dependent on the anterior segment anatomy, the retinal pathology, and vitreous base abnormalities. If the proliferation is primarily posterior, one could opt to leave the lens in place. The presence of the lens does not usually impair posterior dissection techniques, but during fluid–gas exchange, visibility can occasionally be reduced owing to any number of reasons. Air or silicone oil can gain access into the anterior chamber, making adequate optical correction difficult. Condensation on the posterior lens surface can impair the view of the posterior pole, necessitating wiping of the lens with a soft-tipped silicone tube or coating the lens with a viscoelastic substance. It is more common to need to remove anterior chamber lenses than posterior chamber lenses.
The type of intraocular lens present in an eye with PVR may determine the form of long-term retinal tamponade used. The presence of a silicone intraocular lens with an open posterior capsule is a relative contraindication to the use of silicone oil tamponade. Silicone oil adheres irreversibly to the silicone lens surface, which may lead to poor vision and a disrupted view of the retina after attempted silicone oil removal.118 C3F8 gas may be a better choice for such patients.
Vitrectomy
The vitreous cutter is used to perform a core vitrectomy with either standard contact lenses, or more frequently, using a widefield viewing system. A fiberoptic light pipe provides illumination (Fig. 9). Once the core vitrectomy is complete, a prism contact lens or a widefield system is used to remove the peripheral vitreous. This is frequently done in conjunction with scleral indentation to help improve visualization of the peripheral retina and to stabilize the retina during trimming of the vitreous base (Fig. 10). Newer vitrectomy machines with higher cut rates may allow for safer vitreous removal near detached retina. Illumination of the operating field is obtained either through coaxial illumination, fiberoptic endoillumination, or a combination of the two. Once this process has been completed, attention can be turned toward membrane removal.
Membrane Peeling and Sectioning
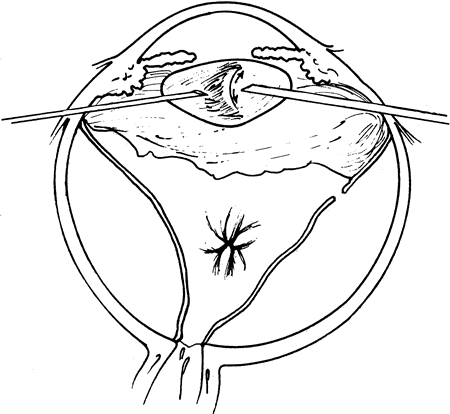
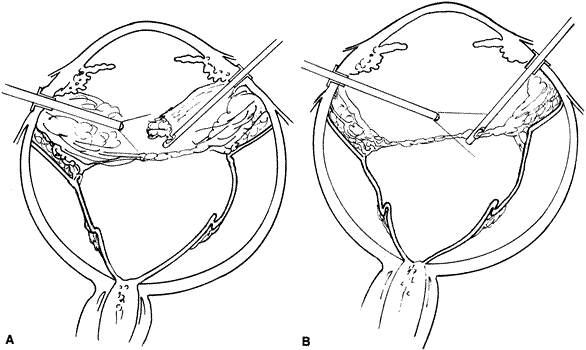
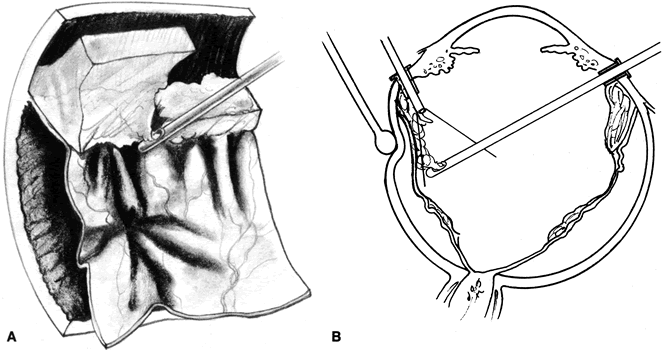
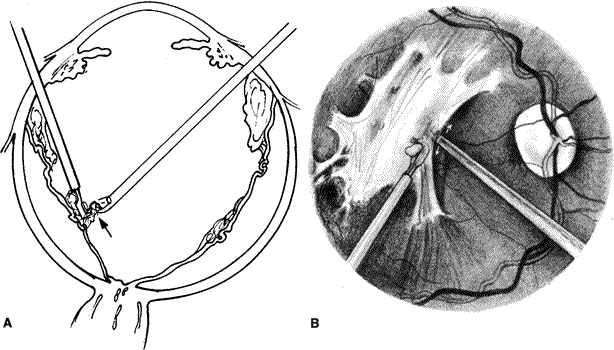
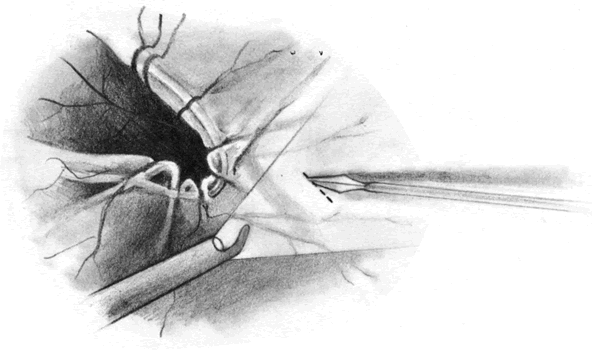
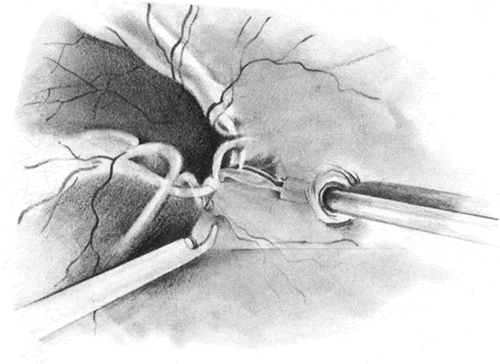
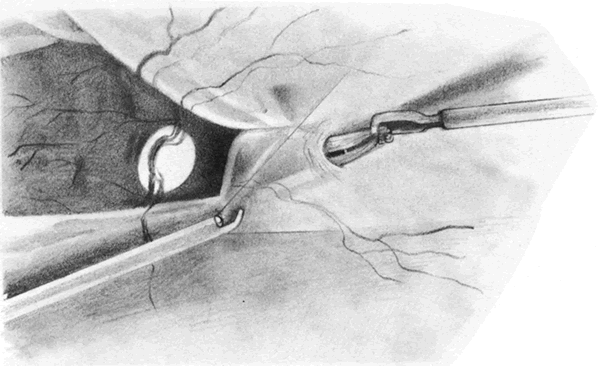
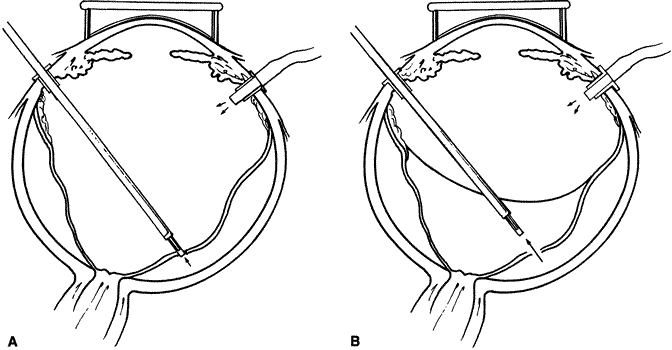
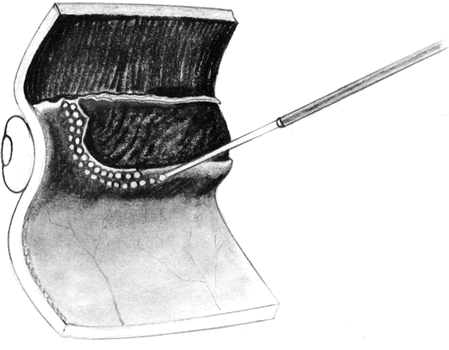
The advent of multifunctional illuminated instruments including illuminated picks and forceps has been of great value in PVR surgery. These instruments have permitted the development of true bimanual dissection techniques, permitting more complete membrane removal and subsequent relief of traction. Also, various forms of chandelier illumination have been developed to allow for bimanual surgery without illuminated instrumentation.119 We prefer to begin the dissection process posteriorly when the retina is not bullous and mobile, although other surgeons prefer to begin membrane removal in the periphery. A fiberoptic pick and an illuminated or nonilluminated microforceps are used to identify and strip membranes in the region of the optic nerve (Fig. 11A). Frequently, the edge of the membrane is identified within 1 mm of the optic disc. Recently, the use of an intravitreal triamcinolone injection has been suggested to improve visualization of the posterior hyaloid during PVR surgery.120,121 The membranes are then stripped anteriorly. Thus most of the stress is applied to the thicker posterior retina rather than the thinner anterior retina. Attempts should be made to strip membranes in large sheets. This usually can be done up to the vitreous base, at which point the membranes become very adherent and complete removal frequently becomes impossible. During the posterior dissection, it is often necessary to pass the pick between folds in the retina to engage the edge of a membrane. In doing so, one must be particularly vigilant not to puncture the retina. If this does occur, the site should be immediately marked with transvitreal diathermy so that it can be later treated with laser or cryopexy. Once an edge of a membrane is elevated, it can be stripped by grasping the membrane with microforceps and sweeping a fiberoptic pick under the membrane to sever its connections to the retina (see Fig. 11B). Alternatively, a micro-forceps and endoilluminator can be placed under the membrane and spread apart. This helps minimize the force directed toward the retinal surface. Once all the traction has been adequately relieved posteriorly, dissection can be continued anteriorly. Once in the periphery, further dissection efforts usually require scleral indentation (Fig. 12A). Care should be taken during the dissection efforts to minimize the number of iatrogenic breaks. The combination of thin retina and very adherent membranes makes this difficult. If a peripheral trough is present owing to anterior displacement of the posterior vitreous base and retina, it should be opened using the microcutter or automated microscissors (seeFig. 12B). The choice of the instrument depends on the width of the trough. Residual vitreous inside the trough is then removed with the microcutter. Membrane removal is continued. A delamination or radial sectioning technique may be required to relieve anteroposterior and circumferential traction (seeFig. 12A). Rarely, the membranes are so tenacious that a 20-gauge knife is required to make radial incisions to relieve circumferential traction. In cases of severe proliferation in the region of the ciliary body, a microforceps or a pick can be used to grasp or elevate the proliferation enough to permit delamination with a pair of microscissors. During this process, one must be careful not to grasp or cut ciliary processes. Cutting of the ciliary body causes bleeding that can be controlled by scleral indentation over the bleeding site, increasing the intraocular pressure, or intraocular diathermy. The use of perfluorocarbon liquid allows intraoperative stabilization of the posterior retina, significantly simplifying extensive anterior dissections (Fig. 13).122
Scleral Buckle
The purpose of a scleral buckle in PVR surgery is to support the region of the vitreous base. When good dissection of the vitreous base has been achieved, a 3.5- to 5.0-mm encircling silicone band provides an adequate scleral buckle. If it has not been technically feasible to relieve all of the peripheral traction, the placement of a broader element such as a no. 287 silicone tire is recommended to more extensively support the region of the vitreous base (Fig. 14).
Fluid–Gas or Fluid–Silicone Oil Exchange
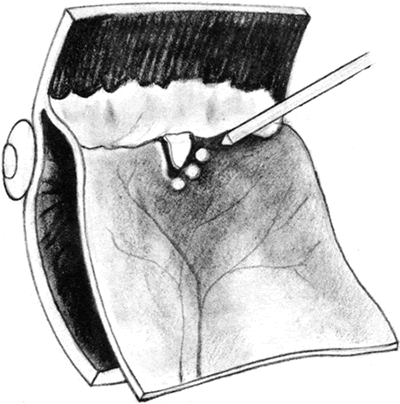
Once the epiretinal proliferations have been adequately removed, a fluid–gas or fluid–silicone oil exchange is performed using a soft silicone-tipped extrusion needle. Frequently, pre-existing posterior breaks permit an adequate exchange. If a retinal break is not already present or is very anterior and perfluorocarbon liquids are unavailable, then a posterior drainage retinotomy may be required. Before performing this maneuver, it is helpful to mark all breaks with transvitreal diathermy to facilitate visualization during retinopexy (Fig. 15). In addition, if silicone oil is to be injected, an inferior peripheral iridectomy should be performed to decrease the likelihood of the oil from causing postoperative pupillary block glaucoma or keratopathy.
|
To perform the fluid–gas or fluid–silicone oil exchange with internal drainage of subretinal fluid, the extrusion needle is placed over the retinal hole, and the gas or silicone oil injection is begun. An automated air injector or silicone oil pump greatly facilitates this procedure. During the exchange, one needs to watch carefully for residual retinal traction (Fig. 16). If the retina looks as if it is being placed on stretch, then the exchange should be immediately halted and the retina examined to determine the source of the traction. Persistent retinal traction can be due to incompletely removed epiretinal proliferations, extensive subretinal proliferation, or retinal foreshortening (Fig. 17). Once the retina has been flattened, endolaser, indirect laser, transscleral laser, endocryopexy or exocryopexy may be applied. In eyes in which a silicone oil tamponade is desired, the use of a sequential fluid–gas, gas–silicone oil exchange versus a direct fluid–silicone oil exchange is dependent on the surgeon's preference.
|
Management of Residual Traction
The most frequent site for residual proliferations is anterior. If these proliferations are preventing the retina from settling, then one must determine whether increasing the height and width of the buckle will relieve the traction. If this is not the case, a relaxing retinotomy will be required. If the proliferation is very focal, a localized buckle or retinectomy may be advantageous.
It is uncommon for subretinal proliferations to be so taut that they prevent the retina from settling. This usually occurs in chronic retinal detachments with severe PVR. They may appear as “clothesline”-like proliferations, diffuse “moth-eaten” membranes, and occasionally as a posterior 360-degree ring of proliferation just anterior to the optic nerve (see Fig. 17).
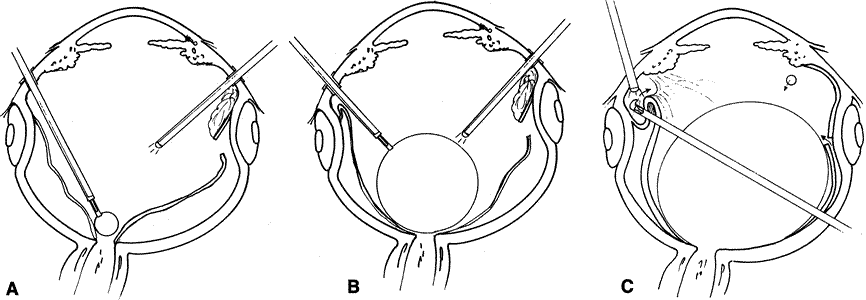
The approach to subretinal proliferations depends on their configuration and extent. Localized bands can be removed by making localized retinotomies at the extreme ends of the band using diathermy or a microvitreoretinal blade (Fig. 18). A retinal micropick is used to partially mobilize the strand, which is then grasped with a pair of microforceps (Fig. 19). The band is placed on traction, and a wiggling motion is used to separate it from its subretinal attachments (Fig. 20). Frequently, under the tension the band will snap and the remaining end will retract toward its proximal end. The remaining proliferation is removed in a similar fashion.
|
Broad sheets of subretinal proliferation may be better approached by means of a large circumferential retinotomy. The retina can then be folded over on itself and the bimanual techniques used to peel the proliferations off of the posterior surface of the retina.
In situations in which the retina appears to be shortened, the surgeon should carefully examine the peripheral retina to determine if a narrow trough caused by anterior displacement has not been opened up. If a tight trough is found, it needs to be opened using scissors and the dissection techniques discussed earlier used (see Fig. 12B). Occasionally, the retina appears to have developed a loss of intrinsic elasticity and will not adequately stretch to line the inside eye wall. If a broad, high buckle is already in place, and additional elements will not relieve the traction, then a relaxing retinotomy is required.
Relaxing Retinotomy
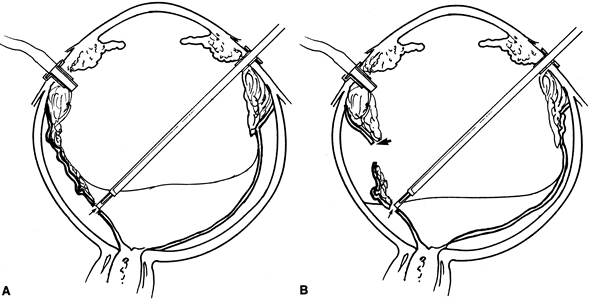
The primary reason for performing a relaxing retinotomy or retinectomy is that retinal traction cannot be adequately relieved with further preretinal membrane dissection or scleral buckling. Rarely, cellular proliferation causes severe contraction and hole formation around an old posterior drainage retinotomy. In these cases, a localized 360-degree retinotomy around the site may be required. If the retina is primarily contracted or residual proliferations cannot be removed, an anterior circumferential relaxing retinotomy is created to relieve the traction. If residual proliferation is present, this is removed along with the underlying retina, together with the devitalized anterior retinal flap. It is very important that no residual traction remains on the retina posterior to the relaxing retinotomy or redetachment is likely.
The location of the planned retinotomy is marked with confluent endodiathermy (Fig. 21A). Large vessels are coagulated approximately 1 mm proximal to the retinotomy to prevent bleeding. The diathermy is continued onto the buckle and attached retina (if present) or until all traction is relieved. A vitreous cutter or microscissors is used to cut the retina anterior to the diathermy marks (see Fig. 21B). The microscissors provide a more controlled cut and can be used to gently lift the retina before cutting to avoid inadvertently cutting the retinal pigment epithelium or choroid. The cut posterior retina is gently mobilized to ensure complete separation from the peripheral retina. The remaining peripheral retina is stripped anteriorly and removed to prevent it from serving as a potential source for recurrent proliferations. After the retinotomy is completed, the posterior surface of the retina is examined for subretinal proliferations. If any are present, they are removed as previously described. The retina is then reattached by fluid–air or fluid–silicone exchange (Fig. 22). The advent of liquid perfluorocarbons as a temporary tamponade has further facilitated this procedure and has made it less traumatic. The perfluorocarbon is then exchanged for gas or silicone oil at the end of the procedure.
Retinal Tamponade
Eyes with PVR require prolonged intraocular tamponade to permit adequate adhesion of the retina to the retinal pigment epithelium. The two most common modalities used as extended intraocular tamponade are intraocular gases (perfluoropropane [C3F8]) and sulfur hexafluoride [SF6]) and silicone oil. A gas tamponade has some advantages over silicone oil. A gas tamponade allows the duration of the tamponade to be varied depending on the concentration and type of gas used (Table 2). Intraocular gas can be supplemented or removed without taking the patient back to the operating room, and gases dissipate spontaneously, thus obviating the need for additional surgery. A nonexpansile concentration of C3F8 (12%123 to 14%124) can last 4 to 8 weeks depending on the aqueous production in the eye. SF6 (18%124 to 20%125) usually only lasts 2 to 3 weeks. The major disadvantages of gas tamponade include the need to adhere to strict positioning requirements, a delay in visual rehabilitation, the absence of a permanent tamponade, and the fact that patients are unable to travel by plane126 until the gas bubble has resorbed.
Table 2. Physical Properties of Commonly Used Gases in PVR Surgery
| Gas | Molecular Weight | Expansion | Longevity (days) | Nonexpanding Concentration |
| Air | 29 | 0 | 5–7 | |
| Sulfur hexafluoride (SF6) | 146 | 2 | 10–14 | 18%–20% |
| Perfluoroethane (C2F6) | 138 | 3.3 | 30–35 | 16% |
| Perfluoropropane (C3F8) | 188 | 4 | 55–65 | 12%–14% |
Data compiled from multiple sources.123–125
Silicone oil is a polysiloxane polymer that has a refractive index of 1.405 and a specific gravity of 0.96 to 0.98 g/cm2. Thus, it floats. Its buoyancy force and surface tension are less than gas. The viscosity of various silicone oils is dependent on their molecular weight, which is directly related to the length of the polymers comprising the oil. The commonly used oils today have viscosity coefficients between 1,000 and 5,000 centistokes. The advantages of silicone oil tamponade include (1) it is a potentially permanent tamponade of constant size; (2) its refractive index permits optical correction, thus speeding visual rehabilitation; (3) it allows for less stringent positioning requirements; and (4) it permits patients to travel by plane. The disadvantages of oil are that it must be removed surgically and that it has a lower surface tension than gas.
Perfluorocarbon Liquids
Perfluorocarbon liquids have specific gravities that are almost twice the density of water, permitting them to generate a tamponade force approaching that of a gas. In addition, they have refractive indices close to saline, high interfacial tensions with water, and low viscosities (2 to 3 centistokes at 25°C). These agents are therefore excellent for tamponading the retina in a supine position and forcing subretinal fluid, including blood, anteriorly through peripheral retinal breaks. Fluorochemicals are also immiscible with silicone oil and blood, enhancing visibility during exchanges.122 The potential for toxicity127–129 is considered by most surgeons to prohibit their use as an extended intraocular tamponade. Consequently, liquid perfluorocarbons are generally used as intraoperative aids, with removal at the end of the procedure.
Retinopexy
There are several modalities available for creating chorioretinal adhesions, including laser (argon, diode), cryopexy, diathermy, and cyanoacrylate tissue adhesive. The latter two are infrequently used in PVR surgery. Laser and cryopexy can be delivered by means of a transvitreal or transscleral approach. Lasers have the added flexibility of delivery via an indirect ophthalmoscope. Endolaser and endocryotherapy are preferred over external cryopexy and are performed only after the retina has been reattached by gas, silicone oil, or liquid perfluorocarbons. In most situations, endolaser is the preferred modality of retinopexy (Fig. 23). However, when the cornea is hazy, air is in the anterior chamber of a pseudophakic eye, or significant subretinal blood is present, endocryopexy is often used. Endocryotherapy is also useful for treating areas on a buckle where a laser cannot be focused or the aiming beam cannot be visualized. The use of transscleral cryotherapy has decreased because of the possible risk of exacerbating PVR. This is believed to occur through breakdown of the blood-ocular barrier130,131 and the propensity for transscleral cryopexy treatments to disperse viable RPE cells into the subretinal and vitreous cavities.90,91
|
Scleral dissection to provide a bed for transscleral diathermy has been recommended to minimize scleral necrosis. It has been suggested that newer models can be used through full-thickness sclera with less risk of scleral burns and thinning.132 Some surgeons believe that transscleral diathermy stimulates PVR less than transscleral cryotherapy. Transscleral diode laser may prove another useful treatment modality in PVR surgery.133
INTRAOPERATIVE COMPLICATIONS
Eyes with severe PVR require extensive manipulations. The surgeon and assistant must be attentive to the location of the intraocular structures. Most of the severe complications and their management have been discussed (e.g., retinal or choroidal bleeding, iatrogenic retinal breaks, and corneal haze owing to epithelial edema or Descemet's folds). Other possible complications include miosis (Fig. 24); the migration of gas or silicone oil into the anterior chamber of a phakic or pseudophakic eye; the subretinal or suprachoroidal infusion of fluid, air, silicone oil, or liquid perfluorocarbons; high intraocular pressure during oil infusion; and foveal injury during laser treatment.
POSTOPERATIVE MANAGEMENT
The typical postoperative regimen includes topical medications such as corticosteroid, cycloplegic, and antibiotic drops. The patients usually wear a patch and shield for 24 hours. Thereafter, the shield is worn all the time unless the patient wears glasses, which can be worn during the day. Modified pressure patching or a bandage contact lens is maintained in patients who have a corneal epithelial defect until the defect is healed.
Most patients undergoing PVR surgery will have some form of intraocular tamponade that necessitates postoperative positioning. The tamponade agents commonly employed are lighter than water and therefore patients must usually assume facedown positioning. Strict positioning is less necessary with silicone oil than with gas. Patients and nursing staff need to be educated on the importance of positioning. In particular, the patient and family should be warned of the risks of patients resting on their back. The most important risks are increased intraocular pressure and cataract.
RESULTS
The skill of vitreoretinal surgeons and the refinement of surgical instrumentation has now made it possible ultimately to reattach many rhegmatogenous retinal detachments complicated by PVR. Despite increasing surgical success, there remains considerable controversy as to which intraocular tamponade is preferable in PVR surgery. In an attempt to help guide vitreoretinal surgeons in their management of patients with severe PVR a multicenter, prospective, randomized, controlled clinical trial comparing the efficacy of a long-acting gas bubble or silicone oil as an intraocular tamponade was begun in 1985 by the Silicone Study Group.134 Patients were eligible for the study if they had PVR of at least grade C-3 (Retina Society classification)63 requiring intraocular dissection, were at least 18 years of age, and had a visual acuity better than “no light perception.” Patients were excluded if their retinal detachment was due to trauma (blunt or penetrating), a giant retinal tear, or proliferative diabetic retinopathy. Patients were also excluded if they had uncontrolled concomitant eye disease or were not likely to be able to be observed for 3 years.135
Patients were stratified into two groups: those who had not undergone a previous vitrectomy for PVR (group 1) and those who had previously failed at least one prior vitrectomy with intraocular gas (group 2). The study was divided into two phases with the first phase comparing 20% SF6 to Dow Corning 1,000-centistoke silicone oil.135 In the second phase of the study, 14% C3F8 was substituted for SF6 because of its longer duration as an effective tamponade (see Table 2).135 Supplemental postoperative fluid–gas exchanges were permitted when needed to prolong the duration of the tamponade in those eyes randomized to gas.135,136
Phase 1
Phase 1 of the trial ran between September 1, 1985, and September 1, 1987, and included data on 101 group 1 and 38 group 2 eyes. Patients were observed up to 3 years after surgery. “Success” was defined as a visual acuity of greater than or equal to 5/200 (1.5/60) and macular attachment (with or without complete retinal attachment posterior to the encircling buckle).
Patients randomized to silicone oil had a greater likelihood of achieving a visual acuity of greater than or equal to 5/200 than those who received SF6 gas. Follow-up during the first year and a half of the study revealed that between 50% and 60% of the eyes receiving silicone oil had visual acuities of 5/200 or better, compared with 30% to 40% of the eyes that received SF6 (P < 0.05). At the 3-year follow-up, the number of eyes with visual acuity of greater than or equal to 5/200 increased for eyes randomized to SF6 and decreased for eyes randomized to silicone oil. Throughout the study the percentage of eyes with light perception to 2/200 (0.6/60) vision was similar between the two treatment modalities but the percentage of eyes with no light perception was greater in the eyes receiving SF6. These results demonstrate that silicone oil tamponade provides an increased chance of achieving a final visual acuity of greater than or equal to 5/200 and a decreased risk of visual acuity of no light perception.
Over the first 2 years of follow-up, the rate of successful anatomic reattachment was higher in eyes randomized to silicone oil than those eyes randomized to SF6 (80% vs. 60%, respectively). If the macula was attached, there was a 75% to 85% chance that the entire posterior pole was attached.
The number of group 2 eyes (previously vitrectomized) in phase 1 of the study were insufficient to permit adequate statistical evaluation. In general, results were similar to group 1, with eyes receiving silicone oil having a higher chance of achieving retinal reattachment or a visual acuity of greater than or equal to 5/200 than eyes receiving SF6.
Phase 2
Phase 2 of the study ran from September 1987 to October 1990 and reported data on 131 group 1 eyes and 134 group 2 eyes. When eyes were randomized between those receiving 14% C3F8 and those receiving silicone oil, no dramatic differences were found between the groups with regard to visual and anatomic “successes.” Forty-three versus 45% and 38% versus 33%, of group 1 and 2 eyes, treated with C3F8 and silicone oil, respectively, had visual acuities of greater than or equal to 5/200. With both C3F8 and silicone oil and in both groups 1 and 2, eyes that were successfully reattached after one operation had significantly better final vision than eyes that required more than one operation to achieve successful anatomic reattachment. For group 1 eyes, the likelihood of achieving a visual acuity of at least 5/200 was 29% in eyes with successful anatomic reattachment requiring multiple operations compared with 78% for eyes that were successfully reattached after one operation. In contrast, for group 2 eyes, 32% had a visual acuity of greater than or equal to 5/200 after randomization surgery and 6% had this visual acuity after one or more reoperations, for a total of 38% visual successes.
At the last follow-up examination, eyes treated with either C3F8 or silicone oil had similar rates of complete posterior attachment (73% vs. 64% for group 1, 73% vs. 61% for group 2). When group 1 eyes with a minimum of 18 months follow-up were evaluated, there was a borderline statistically significant advantage favoring C3F8 over silicone oil in achieving complete posterior retinal reattachment at 18 and 36 months of follow-up. This advantage for C3F8 was not present in group 2.
If one looks at the chances of achieving complete reattachment after one procedure, they are approximately equal (45%) for eyes treated with either C3F8 or silicone oil. The chance of one or more reoperations achieving successful anatomic reattachment was 27% versus 20% for eyes treated with C3F8 and silicone oil, respectively.
The silicone oil study suggests that the operative procedure and the willingness of both the patient and the surgeon for reoperation after initial failure are the primary determinants of anatomic and visual success in PVR surgery rather than whether C3F8 or silicone oil is chosen as the intraocular tamponade. Therefore, at the present time, the major factor in choosing between C3F8 and silicone oil is the preference of the surgeon.
POSTOPERATIVE COMPLICATIONS
Elevated Intraocular Pressure
Transient increases in intraocular pressure (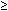 30 mm Hg) are not uncommon in patients who have undergone extensive
surgical manipulations for severe PVR. A single factor or a combination
of several factors may contribute to pressure spikes. Eyes filled
with expansile gases are at risk for increased intraocular pressure, which
can be prolonged and unresponsive to medical management. This situation
is particularly true when the entire intraocular contents have
been replaced by a gas that is still expanding. In these situations, a
pars plana tap or anterior chamber paracentesis (in an aphakic
eye) must be performed to remove some of the gas. Eyes that have
undergone vitreous surgery in conjunction with lensectomy, scleral buckle, and
extensive endolaser procedures are also prone to pressure elevations. This
may be the result of an inflammatory trabeculitis or an
external or internal obstruction of aqueous outflow by a scleral buckle
or inflammatory debris (fibrin and cells). Extensive use of
a laser may cause choroidal swelling, resulting in angle closure due
to anterior rotation of the ciliary body.137 In the past, chronic glaucoma was frequently reported in eyes treated
with extended intraocular tamponade137–140; however, in the Silicone Study, persistent intraocular pressure elevation
was infrequent in eyes treated with gas or with silicone oil (< 1%).135,136
30 mm Hg) are not uncommon in patients who have undergone extensive
surgical manipulations for severe PVR. A single factor or a combination
of several factors may contribute to pressure spikes. Eyes filled
with expansile gases are at risk for increased intraocular pressure, which
can be prolonged and unresponsive to medical management. This situation
is particularly true when the entire intraocular contents have
been replaced by a gas that is still expanding. In these situations, a
pars plana tap or anterior chamber paracentesis (in an aphakic
eye) must be performed to remove some of the gas. Eyes that have
undergone vitreous surgery in conjunction with lensectomy, scleral buckle, and
extensive endolaser procedures are also prone to pressure elevations. This
may be the result of an inflammatory trabeculitis or an
external or internal obstruction of aqueous outflow by a scleral buckle
or inflammatory debris (fibrin and cells). Extensive use of
a laser may cause choroidal swelling, resulting in angle closure due
to anterior rotation of the ciliary body.137 In the past, chronic glaucoma was frequently reported in eyes treated
with extended intraocular tamponade137–140; however, in the Silicone Study, persistent intraocular pressure elevation
was infrequent in eyes treated with gas or with silicone oil (< 1%).135,136
Fibrin
Fibrin formation in the early postoperative period can act as a scaffold for future proliferations and can cause pupillary block glaucoma. Several preoperative and intraoperative factors have been found to be independently predictive of severe fibrin formation after PVR surgery. The preoperative factors include severe anterior chamber flare, poor visual acuity (less than or equal to hand movement), and the presence of a pre-existing scleral buckle. Important intraoperative variables include the performance of extensive anterior dissection and iris manipulations.141 Severe fibrin in the anterior segment resulting in pupillary block can be managed by laser fibrinotomy (argon or yttrium-aluminum-garnet), surgical removal, or, most recently, using intraocular tissue plasminogen activator.142,143 The intraocular injection of 6 to 25 μg of tissue plasminogen activator results in dissolution of the clot within an hour.143,144 Severe posterior fibrin can also be managed with intraocular administration of tissue plasminogen activator, but repeat injections may be required to prevent sheets of fibrin from re-forming on the posterior pole.
Intraocular Hemorrhage
Intraocular hemorrhage in the immediate postoperative period is most frequently seen in eyes that have had extensive relaxing retinotomies.145,146 Occasionally, bleeding may occur during closure of the sclerotomies. In gas-filled eyes, the blood frequently moves into the anterior segment with patient positioning. The blood, if not extensive, will be removed through the trabecular meshwork. If the hemorrhage fails to clear in a reasonable amount of time or hypotony is present (indicating poor aqueous production and turnover), then a postoperative fluid–gas exchange should be considered. In eyes filled with silicone oil, the situation tends to be more problematic. Frequently, the bleeding occurs at the edge of the retinotomy and is confined there by the oil. This tends to occur inferiorly, primarily because this is where the majority of retinotomies are performed.147 Blood layered against the retina appears to stimulate reproliferation and is associated with recurrent retinal detachment.147 If significant periretinal hemorrhage is noted in the early postoperative course, repeat surgery and removal of the blood should be considered.
Hypotony
A common late complication of PVR surgery is that of hypotony (intraocular pressure < 5 mm Hg).135,136,140,148 Up to 25% of group 1 eyes of the Silicone Study that received gas (SF6 or C3F8) developed hypotony. Group 1 eyes that were randomized to silicone oil had a hypotony rate of 10% to 20% (phase 1 and 2 of the study, respectively). The occurrence of hypotony was closely associated with the presence of a detached macula. Eyes with an attached macula had a 5% hypotony rate regardless of the tamponade. In eyes with an attached retina and hypotony, several possible explanations have been reported to explain the low pressure. These include reduced aqueous production due to a reduced blood supply to the ciliary processes secondary to compression or destruction of the long ciliary vessels by a very large buckle or extensive retinopexy, respectively. Alternatively, traction on the ciliary body by anterior proliferation may result in ciliary body atrophy or a cyclodialysis. Hypotony is also common in eyes that have had large pieces of retina removed after a relaxing retinotomy.146 Lastly, inherent toxicity of the various intraocular tamponades may also play a role in the reducing aqueous production. In all likelihood, it is not a single event that results in hypotony but a combination of those just mentioned. Unfortunately, no good treatment exists for chronic hypotony and frequently silicone oil is required to maintain the structural integrity of the globe. Anterior sectioning and removal of the extensive proliferations in the region of the ciliary processes, if performed early, has occasionally been successful in restoring aqueous production.149 It has been our experience that if ciliary processes are noted to be flat and atrophic during the dissection efforts, it is unlikely that aqueous production will be restored.
Keratopathy
Keratopathy (e.g., epithelial or stromal edema, band or bullous keratopathy) is another frequent problem after vitreous surgery for severe PVR. In phase 2, group 1 eyes of the Silicone Study, 33% of eyes that received C3F8 and 30% of those that received silicone oil developed keratopathy by the last follow-up examination. In group 2, an even higher prevalence of keratopathy was noted with 45% of eyes receiving C3F8 gas and 43% of eyes receiving silicone oil noted to have developed keratopathy by the last follow-up. As expected, keratopathy was much more frequent in eyes in which anatomic reattachment failed.135,136
Recurrent Retinal Detachment
Despite successful anatomic reattachment of the retina at the time of vitreous surgery in patients with PVR, recurrent retinal detachments are common.135,136 The primary cause is a continuation of the proliferative process with recurrent growth of membranes. With maturation and contraction of the membranes previously treated, retinal breaks may be reopened or new retinal breaks may occur. In phase 2 of the Silicone Study, recurrent detachments were seen generally 4 to 8 weeks after surgery. Reoperation on these eyes carried a 55% to 71% chance of complete retinal reattachment, but visual results were disappointing.
FUTURE CONSIDERATIONS
The advent of vitreous surgery permitted the reattachment of retinas with severe PVR that were irreparable by conventional scleral buckling procedures. Despite innovations in instrument design, refinements of surgical techniques, and the use of extended intraocular tamponades, about 50% of eyes that have undergone successful primary vitreous surgery for severe PVR suffer recurrent retinal detachments. 135,136 Although repeat operations can completely reattach the retinas of some of these eyes, the likelihood of achieving visual success declines.136 Because of these results, the search for improved therapeutic modalities continues.
Adjunctive Pharmacologic and Gene Therapy
A promising area of research involves the use of pharmacologic agents to inhibit the vitreoretinal scarring response.150 A variety of drugs have been identified that act to inhibit intraocular proliferation. 150–158 These include synthetic peptides,159,160 naturally occurring complex polymeric glycoproteins (heparin),150 anti-inflammatory agents (e.g., triamcinolone acetonide, indomethacin),150,152,161 and synthetic and naturally occurring antimetabolites (e.g., 5-fluorouracil, daunorubicin).150,151,153–157 Clinical trials using some of these agents have shown encouraging preliminary results.156,162–164 A prospective, randomized, controlled trial showed that the combination of 5-fluorouracil and low-molecular-weight heparin added to the intraocular infusion fluid significantly reduced the incidence in postoperative PVR in patients undergoing primary vitrectomy for retinal detachment who were considered at high risk for the development of PVR.165 The role of this combination therapy in patients with pre-existing PVR has not been established.
As our understanding of the pathogenic mechanisms governing the vitreoretinal scarring process grows, the use of targeted drug delivery systems comes closer to reality. For instance, liposomes that can protect enclosed drugs from enzymatic digestion, reduce drug toxicity, and enhance therapeutic effectiveness can be linked to antibodies to provide cell-specific delivery.166,167 Alternatively, the use of targeted immunotherapy, linking antiproliferative agents to site-specific antibodies,158,168–170 or combination therapy using nondegradable implants171 or biodegradable microspheres containing one or more antiproliferative agents may ultimately provide a better treatment for PVR.172,173 An even more targeted approach in treating or preventing PVR involves virus-mediated transfer of inhibitory genetic material into the cells of proliferating tissue.174 Gene therapy has been shown to successfully decrease PVR in animal models by the inhibition of growth factor signaling.175,176 As more is known about the biochemical events that lead to the formation of PVR, more targeted and effective genetic treatments may be possible.