Copious irrigation of the conjunctival sac with sterile saline effectively washes away most debris from the conjunctival surface. Despite irrigation, the conjunctiva usually is colonized by a variety of bacteria. Staphylococcus epidermidis can be cultured in 37% to 70% of patients before scleral buckling.15 More virulent pathogens, such as Staphylococcus aureus or gram-negative bacteria, including Proteus and Pseudomonas, are present in 5% to 9% of routine preoperative cultures. The presence of these pathogens preoperatively increases significantly the incidence of postoperative buckle infections, even with preoperative topical antibiotic treatment for 1 to 2 days.15 Although some surgeons use preoperative topical antibiotics, such as fluoroquinolones, there is no compelling evidence that infection rates are diminished.
After irrigation, the skin surface is dried with sterile gauze. This is particularly important when disposable adhesive drapes are used. The goal of surgical draping is to isolate and protect the surgical field from contamination. Common sources of contamination are the oral and nasal cavities.17 When properly applied, adhesive drapes can effectively seal off these areas from the operative field.
SURGICAL ANATOMY OF CONJUNCTIVA, TENON'S CAPSULE, AND EXTRAOCULAR MUSCLES
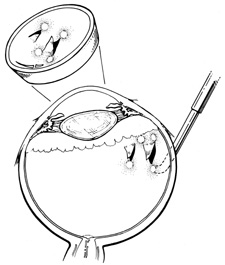
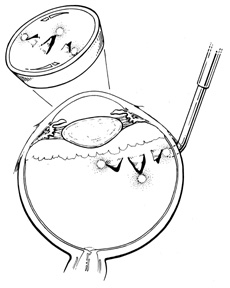
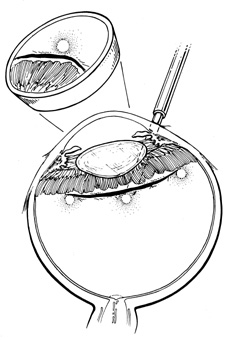
Conjunctival opening can be performed either at the limbus or 4 to 8 mm posterior to the limbus.18,19 Because of the considerable manipulation the conjunctiva undergoes during scleral buckling, radial relaxation incisions are necessary to prevent tearing of the conjunctiva. If a lateral canthotomy is performed, the relaxing incision should be made away from the horizontal meridian to prevent symblepharon formation. The authors routinely use a limbal peritomy and believe this approach allows both better exposure of the sclera and better coverage of the buckle with less postoperative irritation than with a posterior peritomy. In patients with filtering blebs or recent limbal wounds, the peritomy can be extended posteriorly to avoid the area of concern.
Conjunctival opening at the limbus can be facilitated by spreading with scissors beneath Tenon's capsule just posterior to the limbus, thereby avoiding the fusion of conjunctiva and Tenon's capsule, which occurs at the limbus. Once the space between Tenon's capsule and the sclera is entered, conjunctiva and Tenon's capsule can be elevated from the sclera easily and then cut flush at the limbus. With a limbal incision, conjunctiva and Tenon's capsule can be retracted together, usually with less bleeding. If only one or two quadrants are to be buckled, a 360° opening of conjunctiva is not necessary. Conjunctiva and Tenon's capsule can be reflected in the required quadrants only and the appropriate muscles isolated, as described later.
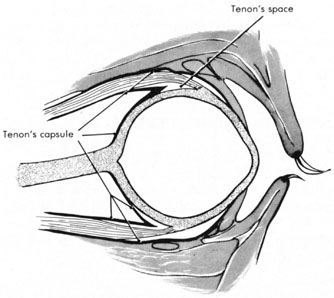
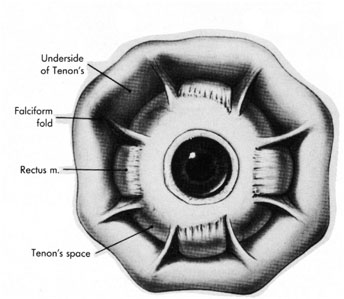
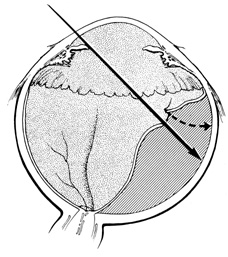
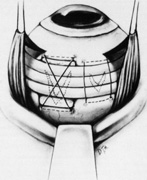
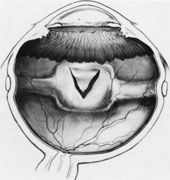
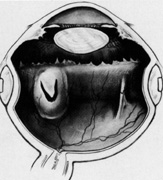
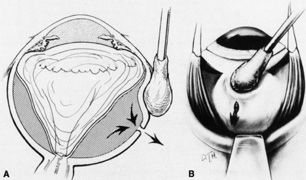
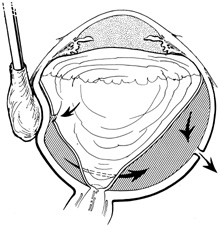
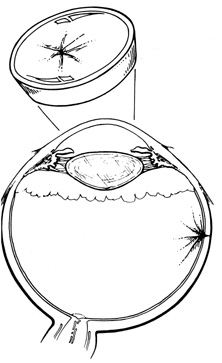
Tenon's capsule is a fascial tissue that invests both the globe and extraocular muscles. Anteriorly it fuses with conjunctiva at the limbus, and posteriorly it ends at the optic nerve sheath. Between Tenon's capsule and the sclera is the interfascial space of Tenon, or simply Tenon's space. Entering this space allows complete exposure of the scleral surface. The extraocular muscles pass through Tenon's capsule, entering Tenon's space to insert on the sclera. At the site of penetration by the individual extraocular muscles, Tenon's capsule reflects posteriorly around the muscles for 10 to 12 mm to form the muscle sheaths. The muscle sheaths are connected by the intermuscular membrane, which, in turn, is connected to the orbital wall by complex fascial arrangements. The retinal surgeon is most concerned with the extraocular muscles after they pass through Tenon's capsule (Fig. 1). At this point they do not possess a muscle sheath but, rather, are invested by episcleral tissue that fuses with the muscle. This tissue forms the falciform folds that fan out from the edges of the muscle to the overlying Tenon's capsule (Fig. 2).
|
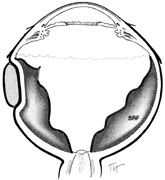
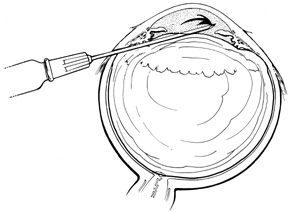
After the peritomy, the space between Tenon's capsule and sclera is entered in the quadrants between the rectus muscles with closed, blunt scissors. Opening the scissors lyses the episcleral fascial connections between Tenon's capsule and sclera. The muscle insertion is then engaged with a muscle hook. The muscle hook should be placed on the sclera and slid posteriorly to the muscle insertion in a circumferential direction. It is then brought anteriorly to engage the insertion. When in the proper space, the muscle hook should glide along the sclera and beneath the muscle with ease. Significant resistance to passage usually means the muscle hook is not on the scleral surface and should be repositioned. The muscle is best engaged by staying anterior to the equator. This also avoids endangering the vortex veins. Once the muscle insertion is engaged, the connections to Tenon's capsule can be identified and separated from the muscle. This is done either by stripping the muscle with a cotton-tipped applicator or with sharp dissection using scissors. When the vertical rectus muscles are isolated, care should be taken not to strip too posteriorly to avoid damaging the levator muscle superiorly or the inferior oblique muscle and Lockwood's ligament inferiorly. After isolation of the muscle is complete, a traction suture is placed around the muscle using either a fenestrated muscle hook20 or a reversed needle; 2-0 black silk is an effective traction suture. All four rectus muscles can be isolated in this manner.
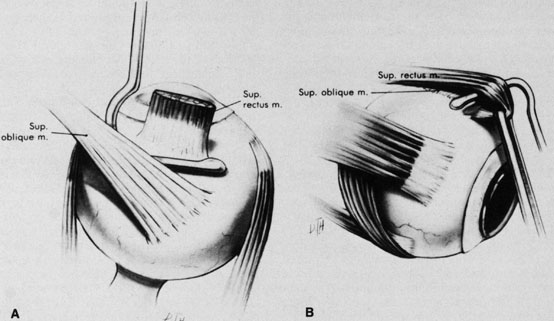
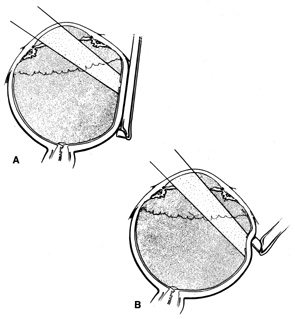
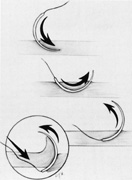
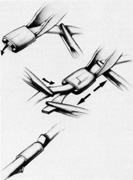
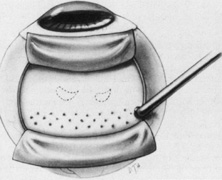
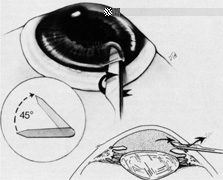
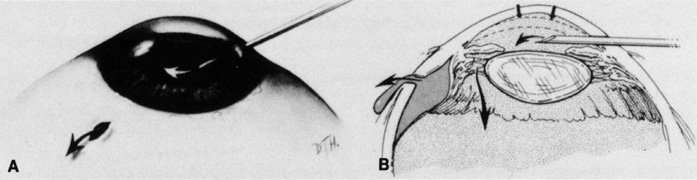
The superior rectus muscle requires additional care to avoid the superior oblique muscle tendon, which inserts 3 to 5 mm posterior to the lateral margin of the superior rectus insertion. Passing the muscle hook from the nasal side anterior to the oblique muscle insertion best avoids engaging the superior oblique muscle tendon. A second method is to rotate the eye inferiorly and grasp the superior rectus muscle insertion with a toothed forceps. The muscle hook then can be passed just posterior to the insertion, thereby avoiding the tendon of the superior oblique (Fig. 3). After the suture is placed around the superior rectus muscle, an inspection for evidence of incarceration of the oblique muscle is made by sweeping posteriorly along the lateral and medial margins of the superior rectus muscle. When incarcerated, the oblique muscle tendon appears as a white band that is pulled anteriorly by the suture.
|
After all rectus muscles are isolated, the surface of the sclera is inspected in each quadrant for evidence of thinning, staphyloma, or anomalous vortex veins, and the location of any abnormalities is noted before scleral depression or marking of retinal breaks. Scleral thinning may appear as a gray-blue area, sometimes associated with obvious ectasia. Less prominent thinning may appear as radial gray lines. Although scleral thinning may occur anywhere, it is most common superotemporally.21
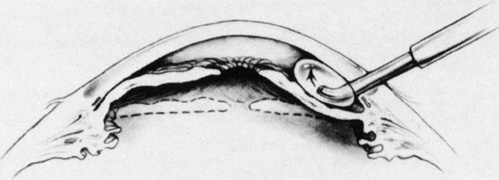
Isolation of the four rectus muscles usually allows adequate access to all areas of the sclera necessary to perform scleral buckling. Rarely, however, a muscle must be removed to adequately expose an area. This usually occurs with breaks beneath muscles or in eyes with tight or small orbits. After the muscle has been isolated, it is elevated with a muscle hook, and a double-armed absorbable synthetic 5-0 or 6-0 suture is passed through the body of the muscle parallel to the insertion and just posterior to the hook. A central knot may be placed. The suture is then passed in a single or double loop through the end of the muscle to occlude the anterior ciliary arteries. With both the suture and hook used for traction, the muscle is elevated from the sclera and cut, leaving a small portion of insertion intact on the sclera. The arms of the suture are secured, and the muscle is allowed to retract posteriorly. A 4-0 silk traction suture is placed through the insertion with at least two passes. After completion of scleral buckling, the muscle is repositioned to the insertion with the double-armed suture (Fig. 4).
|
After isolation of the rectus muscles, the sclera is exposed and accessible for suturing everywhere except beneath the superior oblique muscle and the inferior oblique muscle. Because of its anterosuperior location and wide insertion, the superior oblique muscle more commonly overlies retinal breaks than does the inferior oblique muscle. When this occurs, fibers of either oblique muscle tendon can be cut to expose the underlying sclera. Of course, care should be taken not to entirely disinsert the muscle and also to avoid any underlying vortex veins. Partial resections of the oblique muscle tendons are well tolerated and do not result in motility disorders.
LOCALIZATION
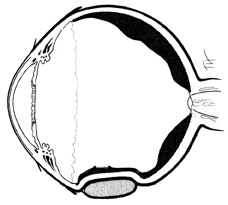
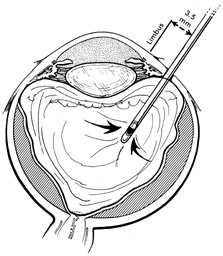
No aspect of scleral buckling is more critical than accurate placement of the buckle on the sclera. This requires precise localization of retinal breaks on the scleral surface. Several instruments for localization and for marking the sclera have been described.22–24 Other surgeons prefer to use the wooden end of a cotton-tipped applicator or a diathermy probe. When the sharper scleral markers are used for localization, it is important to inspect the sclera thoroughly. Sharp localizers and diathermy probes should be avoided if the sclera is thin. The authors prefer the O'Connor localizer, which places a 1-mm circular mark on the sclera with mild indentation (Fig. 5). 24 This instrument also has a smooth surface 90° to the marking surface to allow for exploratory depression before final positioning of the probe. Once the proper site is located, the probe is rotated 90° and the sclera marked with mild indentation pressure for approximately five seconds. The scleral mark is then enhanced with a sterile pen, cautery, or both.
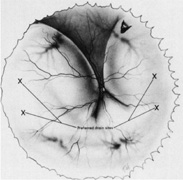
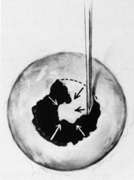
Regardless of which technique or instrument is used for localization and scleral marking, accurate placement of the marks with respect to the intraocular pathology is crucial. For small flap tears or atrophic holes, a single mark on the posterior edge of the break is sufficient. Larger flap tears and nonradial tears require localization of both the anterior and posterior extent of the break (Fig. 6). This anteroposterior orientation is particularly important when radial elements are employed. In areas with multiple, closely spaced tears, it is not necessary to mark each break. Marking the most posterior extent and the circumferential extent of the breaks is adequate (Fig. 7). This approach is also sufficient for marking a retinal dialysis. The circumferential extent of the dialysis is marked anteriorly at the edges of the dialysis, and then the most posterior extent is marked (Fig. 8).
|
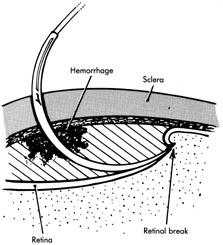
If the retina is bullously detached, accurate localization of retinal breaks is difficult. Bullously elevated breaks appear to lie more posteriorly than their true location because of parallax (Fig. 9). This can result in unnecessarily large and posterior buckles. Inaccurate localization can be minimized by first marking the break at its least elevated margin, usually anterior, and then marking the more elevated margins. Other clues, such as the presence of pigment epithelial changes underlying the break and the location of the ora serrata in relation to the break, may be helpful. Rarely, it may be necessary to drain subretinal fluid to flatten the retina before localization; however, this softens the eye, usually necessitating a saline solution injection to restore volume. It also makes further drainage difficult, presumably because of choroidal swelling secondary to hypotony.
|
TREATMENT OF RETINAL BREAKS
The rationale for the treatment of retinal breaks is to create an adhesion between the retinal pigment epithelium (RPE) and the retina. This is accomplished by inducing a thermal injury with one of three energy sources: diathermy, cryotherapy, or laser. The morphologic and cellular response of the retina and pigment epithelium to each of these energies is essentially similar.25 Two weeks after application, all three methods show comparable effects on retinal adhesive force.26 However, there are significant differences in retinal adhesion immediately after application.26 Photocoagulation increases retinal adhesion within 24 hours of application. Cryopexy reduces retinal adhesion for 1 week after application. Also, there are significant differences in the response the sclera and choroid have to these modalities and in their methods of administration.
Diathermy
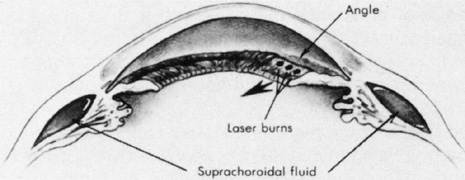
Diathermy is produced by high-frequency, 13.56 MHz, alternating electrical current that generates heat because of the impedance (resistance) of the tissue through which it is passed. The heat created by the diathermy energy coagulates tissues. The histologic picture seen after diathermy depends on the intensity with which it is applied and on whether the retina is attached or detached. When the retina is attached and low-intensity diathermy applied, pigment epithelial migration into the inner retina occurs.27 If the retina is detached, however, low-intensity diathermy treatment results in pigment epithelial proliferation between Bruch's membrane and the external limiting membrane following retinal reattachment.12 High-intensity diathermy treatment in areas of detached retina causes rupture of Bruch's membrane, resulting in fibroblastic proliferation between the choroid and retina when the retina is reattached. The highest-intensity diathermy applications actually rupture the sclera, choroid, and Bruch's membrane, resulting in scar tissue formation across the entire ocular wall.27
Although diathermy produces an effective RPE adhesion, it also induces immediate scleral shrinkage and subsequent scleral necrosis.12,28 This scleral shrinkage results in increased intraocular pressure during diathermy application. The necrosis induced by diathermy weakens the strength of the sclera both immediately after application and over the long term. This weakening complicates reoperation and also may increase the incidence of scleral abscess formation.29 In addition, penetration of diathermy through intact sclera to the retina depends on the scleral thickness. Thus, variations in scleral thickness result in nonuniform and unpredictable transmission of energy to the retina, which can cause choroidal and retinal bleeding and retinal holes.12
The problems of diathermy administration associated with scleral necrosis, shrinkage, and thickness can be minimized by applying it through lamellar scleral flaps, which are discussed later. Transscleral and transconjunctival diathermy without scleral flaps may be administered with a modified diathermy electrode, which causes less scleral damage than conventional diathermy.30 However, because of the full-thickness damage that occurs with diathermy, vortex vein ampullae and long posterior ciliary arteries and nerves must be avoided, even with scleral flaps.12,31 Techniques of diathermy application are discussed in the section on implants.
Cryotherapy
Cryotherapy (cryopexy) produces an effective pigment epithelial–retinal adhesion without the scleral complications that characterize diathermy. This provides cryotherapy with significant advantages: (a) retinal pathologic conditions can be treated without the need for scleral dissection, and (b) retinal breaks can be treated regardless of their location in relation to vortex veins or long posterior ciliary vessels or nerves.
The histologic response following cryotherapy depends on whether the pigment epithelium alone or the pigment epithelium and the overlying detached retina together are frozen.32 The ability to treat detached retina is another significant advantage over both diathermy and photocoagulation. If only the pigment epithelium is frozen without freezing the overlying retina, the pigment epithelial–retinal adhesion that forms once the retina is reattached shows pigment epithelial hyperplasia and loss of retinal outer segments. Therefore the normal microvillous interdigitations seen between retina and pigment epithelium are missing. If both the pigment epithelium and overlying retina are frozen, the adhesion that results after reattachment demonstrates cellular connections between the retina and pigment epithelium consisting of desmosome formation between retinal glia and pigment epithelium or direct contact between retinal glia and Bruch's membrane.
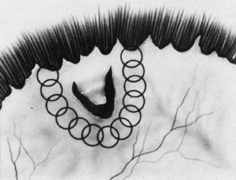
Current cryotherapy instrumentation employs expansion of high-pressure nitrous oxide at the tip of a probe generating temperatures as low as 89°C. The temperature effect is confined to the tip of the probe by an insulating sleeve. A probe 2.0 to 2.5 mm in diameter usually is used for retinal work. Treatment of retinal breaks and pathologic conditions requires accurate placement of the cryoprobe tip. The surgeon must be certain that the indentation visualized with the indirect ophthalmoscope is the tip of the probe and not the shaft. Confusion between the tip and the shaft of the cryo-probe can cause inadvertent posterior freezes.33 To minimize the possibility of this complication, the surgeon must indent only with the tip of the cryoprobe (Fig. 10). It is also helpful to perform the first freezes at the most anterior aspect of the area requiring treatment to assess both location and intensity of treatment.
|
The goal of treatment is to surround all retinal breaks with 1 to 2 mm of contiguous treatment. When possible, treatment should include freezing of the overlying retina, because this results in a stronger adhesion than does treatment of the pigment epithelium alone.25 To avoid the damage of refreezing, treatment should not significantly overlap. The treatment end point is retinal whitening without ice crystal formation.34 Slight whitening of the retina because of retinal edema is noted several minutes after freezing, which helps to assess the adequacy of treatment. If retinal treatment is impossible because of bullous retinal elevation, treatment of the pigment epithelium alone may be performed, or treatment can be deferred until after drainage of subretinal fluid.
For flap retinal tears, treatment is performed contiguously around the tear and then extended anteriorly to the ora serrata. Care is taken not to freeze bare RPE in the bed of the retinal break where there is no overlying retinal tissue. Small retinal breaks and atrophic retinal holes can be treated with single freezes centered on the retinal break (Fig. 11).
One potential disadvantage of cryopexy is dispersion of pigment epithelial cells, which can result in subretinal pigmentary changes after reattachment.35,36 Also, dispersion of viable pigment epithelial cells capable of causing proliferative vitreoretinopathy has been demonstrated following cryopexy.37 The clinical relevance of cryopexy-induced pigment epithelial cell dispersion in the pathogenesis of proliferative vitreoretinopathy following scleral buckling is controversial. Some retrospective analyses suggest that cryopexy is a risk factor for the development of postoperative proliferative vitreoretinopathy (PVR),38,39 whereas other studies do not show an association between cryopexy and PVR.40 Accordingly, it seems prudent to minimize cryotherapy-induced pigment epithelial cell dispersion by not over treating and by avoiding unnecessary scleral depression of treated areas, which enhances dispersion of viable cells.41 Therefore localization and examination with scleral depression should be performed before cryopexy.
In addition, although cryopexy does not permanently damage the choroid, it does induce choroidal congestion and hyperemia,27 which may complicate drainage of subretinal fluid through treated areas. Finally, cryopexy causes breakdown of the blood–ocular barrier,42 and its use has been implicated as a cause of postoperative cystoid macular edema and exudative detachments after scleral buckling.43 Despite these potential problems, cryopexy remains the choice of most retinal surgeons for the intraoperative treatment of retinal breaks during scleral buckling.44
Photocoagulation
Photocoagulation is another means for creating pigment epithelial–retinal adhesion. Laser delivery systems coupled to an indirect ophthalmoscope allow retinal surgeons to use photocoagulation during scleral buckling.45 Laser photocoagulation can also be applied postoperatively after retinal reattachment.46 The pigment epithelium is the primary chromophore for energy uptake with photocoagulation. Therefore the area of retina to be treated must be either attached or placed in juxtaposition to the pigment epithelium by scleral depression. As a result, treatment of bullously elevated retinal breaks or areas with atrophic or attenuated pigment epithelium may be difficult. Intraoperative transpupillary photocoagulation also requires optimal visualization. Miosis, corneal opacification, cataract, and vitreous hemorrhage may preclude transpupillary photocoagulation.
Transscleral diode laser photocoagulation also may be used to treat retinal pathology.47 The long wavelength (810–840 nm) of the diode laser penetrates the sclera and is absorbed by the pigment epithelium. Placing the detached retina in juxtaposition to the pigment epithelium with scleral depression allows treatment of retinal pathology. The desired end point is a gray to gray-white burn. Potential complications include rupture of Bruch's membrane and the pigment epithelium, which may result in choroidal bleeding. Mild thermal effects on the sclera may be evidenced by blue-gray discoloration of the sclera. A potential advantage of transscleral diode photocoagulation over other methods is the ability to treat through preexisting scleral buckling components.48 A disadvantage of both transscleral and transpupillary photocoagulation is that photocoagulation is more time consuming and requires more individual treatment spots than cryopexy.
Photocoagulation has several desirable features. It can be delivered with great precision, both in terms of location and intensity. Compared with diathermy and cryopexy, it causes less breakdown of the blood–ocular barrier.42 The thermal effect of photocoagulation is confined predominantly to the retina and pigment epithelium, with little or no effect on the choroid or sclera.49 Finally, photocoagulation induces an adhesive effect between the retina and pigment epithelium within 24 hours.50