* Metric equivalent given in parenthese after Snellen notation.
SMALL RETINOTOMY TECHNIQUE
In January 1991, Thomas and Kaplan14 reported an alternative approach to subfoveal neovascularization in POHS. Instead of a large flap retinotomy, their technique employed a small retinal hole through which instruments were introduced into the subretinal space. The neovascular membrane was dislodged, grasped with forceps, and extracted through the slightly enlarged retinotomy. An air-fluid exchange was followed by endolaser burns around the retinotomy and short-term tamponade with sulfur hexafluoride gas. In the first two POHS cases, visual acuity improved dramatically (from 20/400 [6/120] to 20/20 [6/6] in one case and 20/400 [6/120] to 20/40 [6/12] in the second). These early encouraging results prompted refinement of the instrumentation and surgical technique and their application in a wider variety of cases.15
CURRENT SURGICAL TECHNIQUE
The surgical technique is still in evolution. The present status is described in this chapter together with a summary of the results achieved after removal of subretinal neovascular membranes of various etiologies. This approach is most effective in those cases in which the membrane lies predominantly anterior to the RPE and thus can be removed without extracting large areas of the epithelium. Preservation of foveal RPE appears to be a critical factor in regaining excellent central visual function.
In most cases that meet the criteria for subretinal surgery, the edge of the neovascular complex can be readily visualized under the operating microscope without angiography. In some relatively recent membranes, even if they are anterior to the RPE, we have found the edges to be more difficult to discern. Thus, on occasion, it is helpful to select a frame from the preoperative fluorescein angiogram to project on a screen in the operating room. The image is inverted and reversed to match the surgeon's view through the operating microscope at the top of the patient's head.
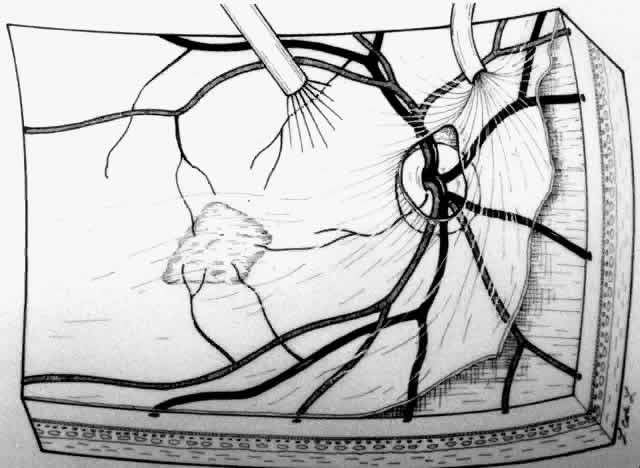
A standard three-port approach is used to carry out a complete pars plana vitrectomy. The placement of the sclerotomy is critical. The surgeon should study the angiogram and decide preoperatively where the retinotomy is to be placed to avoid damaging major vessels, to provide adequate access to the subretinal membrane, and to minimize the size of the scotoma. These factors usually dictate that the retinotomy be created in a straight temporal location and thus the superotemporal sclerotomy should be made near the horizontal meridian. If a sewn-on ring system is used to hold a corneal contact lens, it is sometimes advantageous to rotate the fixation flanges superotemporally and inferonasally from the horizontal to allow a nearly horizontal placement of the temporal port. Occasionally these horizontal sclerotomy sites bleed more than when placed more superiorly, but this has not proven to be a significant complication.
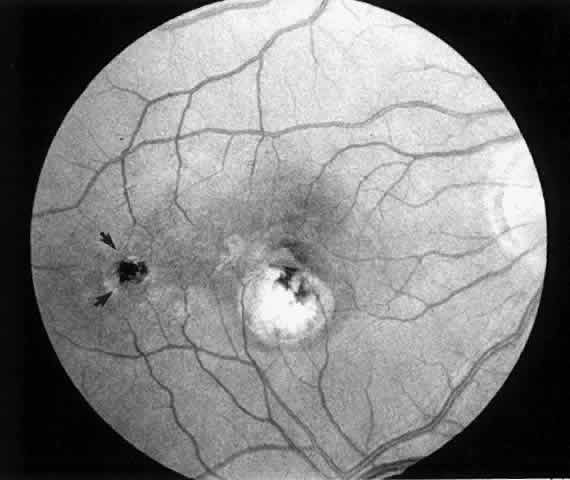
Although data are lacking to prove the importance of removal of the posterior hyaloid, we attempt to remove it in every case. Our preferred technique is using a silicone-tipped extrusion needle to aspirate over the attached cortical vitreous near the optic disc.16,17 The surgical assistant or technician indicates whether infusion fluid is dripping into the collection chamber. Absence of dripping indicates that the silicone tip has engaged the posterior hyaloid, and at that point the aspiration pressure can be raised to 300 or 400 mm Hg. Once engaged, the hyaloid can usually be pulled free from the disc with a gentle stripping motion. In many cases, the surgeon can see a Weiss ring as the hyaloid detaches and is stripped approximately to the equator (Fig. 1). (If the hyaloid remains adherent, then one may proceed with the case. After the creation of the retinotomy, an angled needle tip or the subretinal pick can often be used to find the cleavage plane between posterior hyaloid and retina at the edge of the retinotomy and achieve detachment of the hyaloid.) The vitreous cutter is reintroduced and the vitrectomy is completed.
|
The placement of the retinotomy takes into account (1) the exact location of the membrane under the fovea; (2) the presence of presumed adhesions between the neurosensory retina and underlying tissue (previous photocoagulation scars and/or evidence of pigment migration into neurosensory retina or retinochoroidal vascular anastomoses); (3) the dimensions of the subretinal instruments (specifically the length of the angled instrument tips that determines how far away from the fovea the retinotomy can be made and still allow the tips to reach the membrane); and (4) the topographic anatomy of the neurosensory retina and nerve fiber layer. (A retinotomy made temporally in the macula disrupts less nerve fibers than does a retinotomy made nasally or superiorly that risks the creation of an arcuate visual field defect or reduced central acuity secondary to the interruption of the papillomacular bundle.) In most cases, these factors dictate a straight temporal or slightly superotemporal location for the retinotomy. However, the surgeon may choose to create a retinotomy superonasal to the fovea. With newer 33- and 36-gauge instruments, the retinotomies are small enough that no significant damage to the papillomacular bundle occurs. This approach may allow the surgeon to use his or her dominant hand for subretinal manipulation.
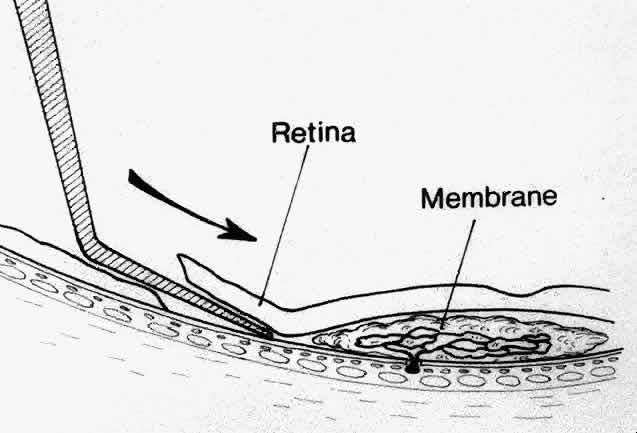
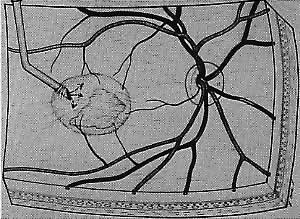
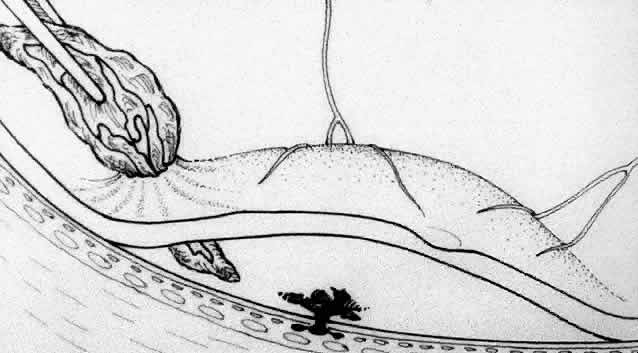
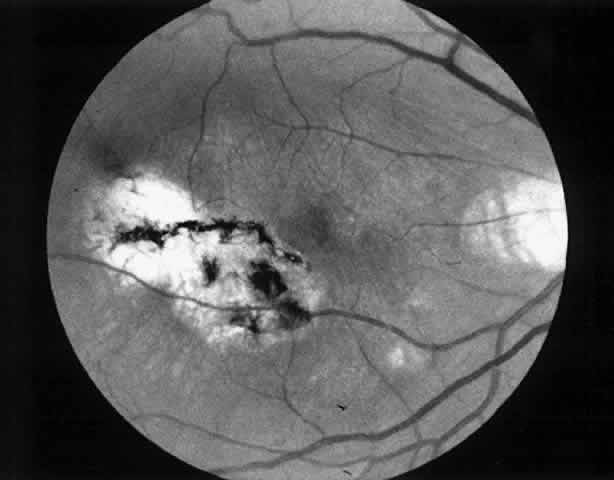
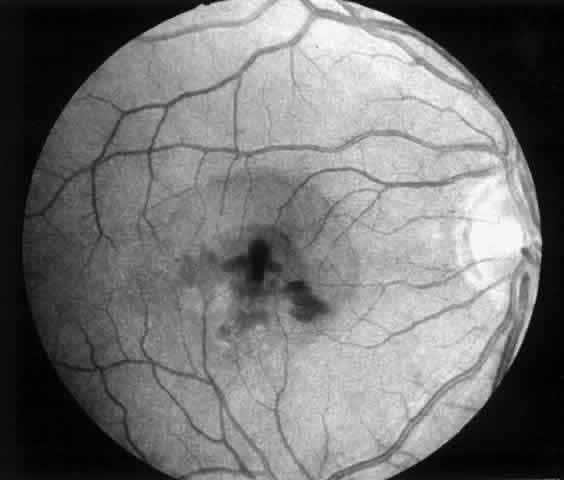
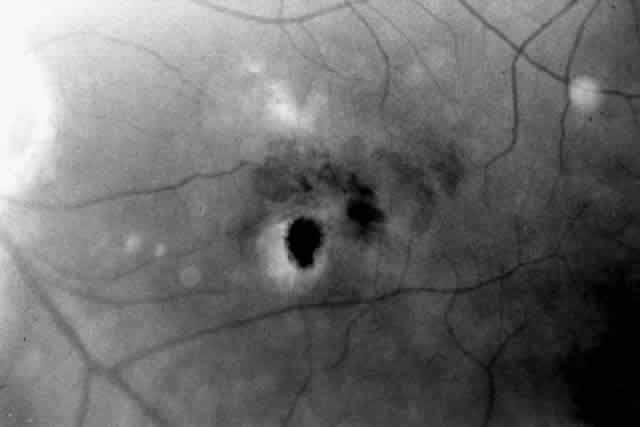
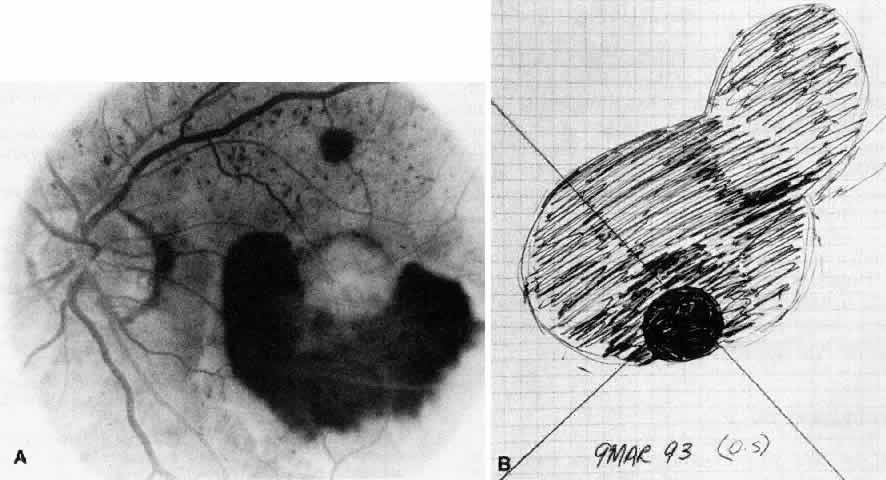
Besides being in the most advantageous location, the retinotomy should be as small as possible. Initially, we lightly diathermized the surface of the retina and then used the myringotomy blade to tease open a small hole through which an angled infusion needle was introduced.15 At the suggestion of Lambert and co-workers at Emory University, we stopped using diathermy. We now use a 120° angled, sharply pointed 36-gauge subretinal pick to pierce undiathermized neurosensory retina (Fig. 2). Occasional slight retinal hemorrhage can be controlled by transiently increasing the intraocular pressure. After the tiny hole has been made, the surgeon introduces the angled 33-gauge infusion needle beneath the retina and the assistant gently infuses balanced salt solution to elevate the neurosensory retina. This is accomplished by pushing on the plunger of a syringe that is connected to the hub of the needle by a short piece of tubing. As the fluid enters the subretinal space, attention is directed to edges of laser scars and/or adhesions to the underlying membrane (Fig. 3). Excessive infusion pressure can easily tear the retina. If areas of retina remain adherent, the infusion is stopped and the tip of an angled subretinal pick is carefully passed over the anterior surface of the membrane surface to break any residual adhesions. In a similar manner, the tip of the angled subretinal pick can be used to gently separate the thinned retina from an underlying photocoagulation scar. Occasionally, horizontal subretinal scissors are necessary to cut firm adhesions. These scissors have a similar 130° bend and blades approximately 3 mm in length to allow manipulation through an eccentric retinotomy. Trauma to foveal photoreceptors from either the pick or scissors is carefully avoided. If the retina is not mobilized over the entire photocoagulation scar, separation is achieved at least far enough into the scar to allow manipulation and extraction of the membrane without tearing the adjacent retina. The sharp tip of the angled subretinal pick is used to elevate the edge of the neovascular complex from the underlying RPE (Fig. 4). Care is taken to swing the pick in a pivoting or rotating manner to stretch or enlarge the retinotomy as little as possible. This requires close attention not only to the primary site of action at the membrane but also to the instrument shaft at the retinotomy site. In the appropriate cases, the complex dislodges easily from the underlying subfoveal RPE but remains attached to the edge of a laser scar (in recurrent cases) or to the stalk of choroidal vascular ingrowth.
|
|
|
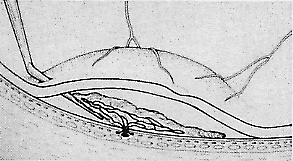
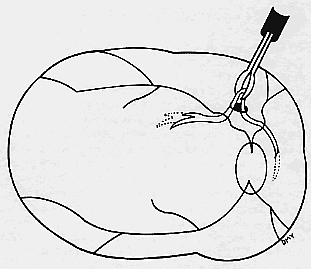
We use positive action horizontal forceps that are angled 130° and have tips 3.2 mm in length.15 The tips are introduced (closed) through the retinotomy, which has usually enlarged slightly during the subretinal manipulation. The objective is to place the opened blades around the stalk or the adhesion, with the membrane in front of the blades. Gentle traction with the blades held closed breaks the connection (Fig. 5). This step is performed slowly and carefully. If traction on the retina is seen, the membrane is released and further separation of the complex from neurosensory retina is accomplished. If excessive tugging and displacement of surrounding RPE is seen, then consideration is given to using the subretinal scissors to cut the stalk rather than breaking it with the forceps. In virtually every case, the membrane (and often the adjacent laser scar) can be removed in one piece. As pathologic examination has confirmed, the abundance of cohesive basement membrane material matrix surrounding occasional capillaries, creates a complex of significant tensile strength.14
|
When the vascular connection from the choroid is about to be severed, the intraocular pressure is raised to approximately 80 mm/Hg. Despite this precaution, minimal hemorrhage is often countered when the membrane is removed. In one early case, while attention was directed to removing the neovascular complex through the sclerotomy, a massive hemorrhage occurred beneath the retina. In two additional cases, enough blood accumulated in the subretinal space to require removal with subretinal forceps. We now maintain the intraocular pressure elevated for at least 1 minute and watch closely for any evidence of rebleeding while the pressure is slowly lowered. If more bleeding occurs, the intraocular pressure is raised again. After the implementation of these measures, significant subretinal hemorrhage has not occurred in the last 60 cases.
Once hemostasis is achieved, the membrane is extracted through the sclerotomy for pathologic examination or it is cut and aspirated with the vitrectomy probe. Occasionally, large membranes cannot be removed through a standard size sclerotomy. We prefer dividing the membrane into smaller pieces with intraocular scissors rather than enlarging the sclerotomy. Plugs are placed and scleral depression is performed 360° to verify that no peripheral retinal tears have occurred.
A complete air-fluid exchange is next performed. We use standard extrusion needles or silicone-tipped needles for the exchange, with the aspirating tip over the optic nerve. In early cases, once the retina was flat, endolaser burns were applied around the retinotomy. The resulting laser scars were eccentric from the fovea but did occasionally produce symptomatic scotomas. In the past 60 cases, we have used no laser and have found the retina to remain attached. Our use of intraocular tamponade has also undergone evolution. Initially, we used nonexpansile concentrations of sulfur hexafluoride or perfluoropropane and encouraged facedown positioning for 1 to 2 weeks. As improved instrumentation allowed smaller retinotomies, we have questioned the need for gas tamponade. We perform fluid-air exchange and leave the eye with a one-half to two-thirds fill of filtered air. In the most recent 15 cases, we have gently reinfused balanced salt solution over the optic nerve after the vitreous cavity has been dry for a few minutes. The eye is completely filled with fluid, and no special positioning is used. In each of these cases, the posterior hyaloid has been meticulously removed. In each case the retinotomy has sealed without gas tamponade. Additional cases will be required before this modification can be widely advised.