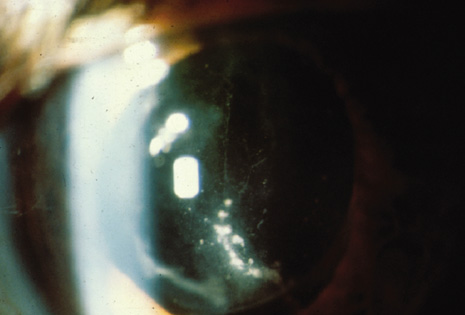
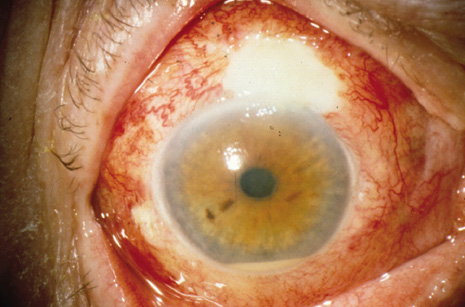
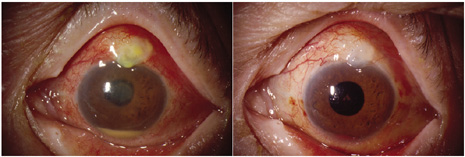
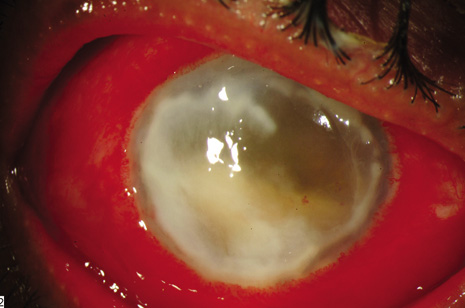
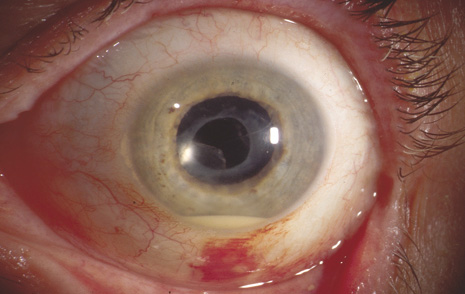
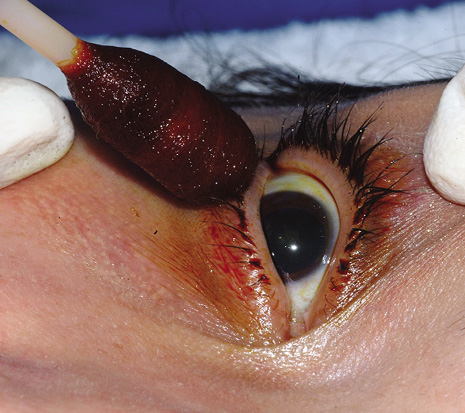
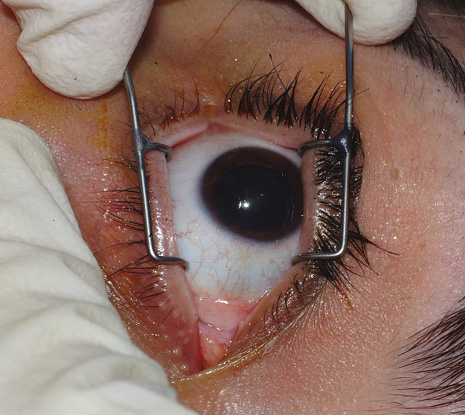
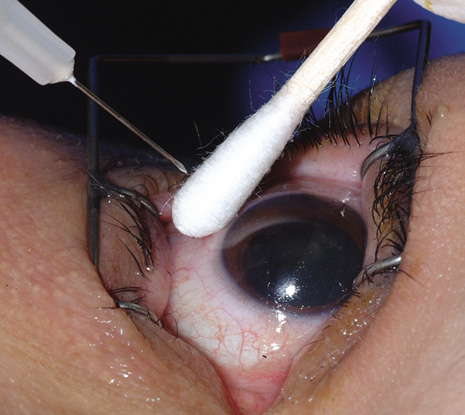
1. American Academy of Ophthalmology: Basic and Clinical Science Course: Section 12: Retina and Vitreous, 2004–5. San Francisco: American Academy of Ophthalmology, 2005:328–331. 2. Doft B. Endophthalmitis Management. Focal Points: Modules for Clinicians. San Francisco: American Academy of Ophthalmology, 1997. 3. Scott IU, Flynn HW Jr, Feuer W, et al: Endophthalmitis associated with microbial keratitis. Ophthalmology 103:1864–1870, 1996. 4. Moshfeghi DM, Kaiser PK, Scott IU, et al: Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol 13: 791–796, 2003. 5. Gelender H: Bacterial endophthalmitis following cutting of sutures after cataract surgery. Am J Ophthalmol 94:528, 1982. 6. Eifrig CWG, Flynn HW Jr, Scott IU, et al: Acute-onset postoperative endophthalmitis: review of incidence and
visual outcomes. Ophthalmic Surg Lasers 33:373–378, 2002. 7. Phillips WB, Tasman WS: Postoperative endophthalmitis in association with diabetes mellitus. Ophthalmology 101:508, 1994. 8. Song A, Scott IU, Flynn HW Jr, et al: Delayed-onset bleb-associated endophthalmitis. Clinical features
and visual acuity outcomes. Ophthalmology 109:985–991, 2002. 9. Mandelbaum S, Forster RK, Gelender H, et al: Late onset endophthalmitis associated with filtering blebs. Ophthalmology 92: 964, 1985. 10. Wolner B, Liebmann JM, Sassani JW, et al: Late bleb-related endophthalmitis after trabeculectomy with adjunctive 5-fluorouracil. Ophthalmology 98:1053, 1991. 11. Brown RH, Yang LH, Walker SD, et al: Treatment of bleb infection after glaucoma filtering surgery. Arch Ophthalmol 112:57, 1994. 12. Ruiz RS, Teeters VW: The vitreous wick syndrome: A late complication following cataract extraction. Am J Ophthalmol 70:483, 1970. 13. Fox GM, Joondeph BC, Flynn HW Jr, et al: Delayed onset pseudophakic endophthalmitis. Am J Ophthalmol 111:163, 1991. 14. Clark WL, Kaiser PK, Flynn HW Jr, et al: Treatment strategies and visual acuity outcomes in chronic postoperative P. acnes endophthalmitis. Ophthalmology 6:1665–1670, 1999. 15. Aldave AJ, Stein JD, Deramo VA, et al: Treatment strategies for postoperative Propionibacterium acnes endophthalmitis. Ophthalmology 106:2395–401, 1999. 16. Ciulla TA. Update on acute and chronic endophthalmitis. Ophthalmology 106:2237–2238, 1999. 17. Puliafito CA, Baker AS, Haaf J, et al: Infectious endophthalmitis: Review of 36 cases. Ophthalmology 89:921, 1982. 18. Rowsey JJ, Newsom MS, Sexton DJ, et al: Endophthalmitis: Current approaches. Ophthalmology 89:1055, 1982. 19. Bohigian GM, Olk RJ: Factors associated with a poor visual result in endophthalmitis. Am J Ophthalmol 101:332, 1986. 20. Brinton GS, Topping TM, Hyndiuk RA, et al: Posttraumatic endophthalmitis. Arch Ophthalmol 102:547, 1984. 21. Thompson JT, Parver LM, Enger C, et al: Endophthalmitis after penetrating ocular injuries with retained intraocular
foreign bodies. Ophthalmology 100:1468, 1993. 22. Thompson WS, Rubsamen PE, Flynn HW Jr, et al: Endophthalmitis after penetrating trauma. Risk factors and visual acuity
outcomes. Ophthalmology 102:1696–1701, 1995. 23. Greenwald MJ, Wohl LG, Sell CH: Metastatic bacterial endophthalmitis: A contemporary reappraisal. Surv Ophthalmol 31:81, 1986. 24. Brod RD, Flynn HW Jr, Clarkson JG, et al: Endogenous Candida endophthalmitis: management without intravenous amhotericin
B. Ophthalmology 97:666, 1990. 25. Weishaar P, Flynn HW Jr, Murray TG, et al. Endogenous Aspergillus endophthalmitis. Clinical features and treatment
outcomes. Ophthalmology 105:57–65, 1998. 26. Okada AA, Johnson RP, Liles C, et al. Endogenous bacterial endophthalmitis. Ophthalmology 101:832–838, 1994. 27. Schiedler V, Scott IU, Flynn HW Jr, et al: Culture-proven endogenous endophthalmitis: clinical features and
visual acuity outcomes. Am J Ophthalmol 137:725–731, 2004. 28. Moshfeghi A, Scott IU, Flynn HW Jr, et al: Pseudohypopyon after intravitreal triamcinolone acetonide injection for
cystoid macular edema. Am J Ophthalmol 138(3)489–492, 2004. 29. Jager RD, Aiello LP, Patel SC, et al: Risks of intravitreal injection: A comprehensive review. Retina 24:676–698, 2004. 30. Sutter FKP, Gillies MC. Pseudo-endophthalmitis after intravitreal injection of triamcinolone. Br J Ophthalmol 87:972–974, 2003. 31. Roth DB, Chieh J, Spirn MJ, et al: Noninfectious endophthalmitis associated with intravitreal triamcinolone
injection. Arch Ophthalmol 121:1279–1282, 2003. 32. Nelson ML, Tennant MTS, Sivalingam A, et al: Infectious and presumed noninfectious endophthalmitis after intravitreal
triamcinolone acetonide injection. Retina 23:686–691, 2003. 33. Jonas JB, Kreissig I, Degenring RF. Endophthalmitis after intravitreal injection of traimcionlone acetonide. Arch Ophthalmol 121:1663–1664, 2003. 34. Chen SD, Lockhead J, McDonald B, et al: Pseudohypopyon after intravitreal triamcinolone injection for the treatment
of pseudophakic cystoid macular edema. Br J Ophthalmol 88(6):843–844, 2003 35. Packer AJ, Weingeist TA, Abrams GW: Retinal periphlebitis as an early sign of bacterial endophthalmitis. Am J Ophthalmol 96:66, 1983. 36. Donahue SP, Kowalski RP, Jewart BH, et al: Vitreous cultures in suspected endophthalmitis: Biopsy or vitrectomy? Ophthalmology 100:452, 1993. 37. Joondeph BC, Flynn HW Jr, Miller D, et al: A new culture method for infectious endophthalmitis. Arch Ophthalmol 107: 1334, 1989. 38. Dworkin LL, Gibler TM, Van Gelder RN: Real-time quantitative polymerase chain reaction diagnosis of infectious
posterior uveitis. Arch Ophthalmol 120:1534–1539, 2002. 39. Wittwer CT, Kusukawa N: Real-time PCR. In: Persing DH (ed): Molecular Microbiology: Diagnostic Principles and Practice. Washington, D.C.: ASM Press, 2004:71–84. 40. Anand AR, Madhaven HN, Neela V, et al: Use of polymerase chain reaction in the diagnosis of fungal endophthalmitis. Ophthalmology 108:326–330, 2001. 41. Chandler PA: Problems in the diagnosis and treatment of lens-induced uveitis
and glaucoma. Arch Ophthalmol 60:828, 1958. 42. Scott IU, Flynn HW Jr. Smiddy WE, et al: Clinical features and outcomes of pars plana vitrectomy in patients with
retained lens fragments. Ophthalmology 110:1567–1572, 2003. 43. Irvine WD, Flynn HW Jr, Murray TG, et al: Retained lens fragments after phacoemulsification manifesting as marked
intraocular inflammation with hypopyon. Am J Ophthalmol 114:610, 1992. 44. Piest TK, Kincaid MC, Tetz MR, et al: Localized endophthalmitis: a newly described cause of the so-called
toxic lens syndrome. J Cataract Refract Surg 13:498–510, 1987. 45. Monsen MC, Mamalis N, Olsen RJ. Toxic anterior segment inflammation following cataract surgery. J Cataract Refract Surg 18:184–189, 1992. 46. Kritza K, Martin S, Young C, et al: Localized inflammation following cataract extraction caused by bacterial
contamination of a cleaning bath detergent. J Cataract Refract Surg 18:106–110, 1992. 47. Jehan FS, Mamalis N, Spencer TS, et al. Postoperative sterile endophthalmitis (TASS) associated with
the memory lens. J Cataract Refract Surg 26(12):1773–1777, 2000 48. Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of
immediate vitrectomy and of intravenous antibiotics for the treatment
of postoperative bacterial endophthalmitis. Arch Ophthalmol 13:1479–1496, 1995. 49. Doft DH, Wisnisky SR, Kellsy SF, et al: Diabetes and postoperative endophthalmitis in the Endophthalmitis Vitrectomy
Study. Arch Ophthalmol 119:650–656, 2001. 50. Endophthalmitis Vitrectomy Study Group: Microbiologic factors and visual outcome in the Endophthalmitis Vitrectomy
Study. Am J Ophthalmol 122:830–846, 1996. 51. Bannerman TL, Rhoden DL, McAllister M, et al: The source of coagulase-negative staphylococci in the Endophthalmitis
Vitrectomy Study. A comparison of eyelid and intraocular isolates
using pulsed-field gel electrophoresis. Arch Ophthalmol 115(3):357–361, 1997. 52. Braza M, Pavan PR, Doft BH, et al: Evaluation of microbiological diagnostic techniques in postoperative endophthalmitis
in the Endophthalmitis Vitrectomy Study. Arch Ophthalmol 116(9):1142–1150, 1997. 53. Doft BH, Kelsey SF, Wisniewski SR, et al: Additional procedures after the initial vitrectomy or tap-biopsy
in the Endophthalmitis Vitrectomy Study. Ophthalmology 105(4):707–716, 1998. 54. Han DP, Wisniewski SR, Wilson LA, et al: Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis
Vitrectomy Study. Am J Ophthalmol 122(1):1–17, 1996. [Published erratum appears in Am J Ophthalmol 122(6):920, 1996.] 55. Han DP, Wisniewski SR, Kelsey SF, et al: Microbiologic yields and complication rates of vitreous needle aspiration
versus mechanized vitreous biopsy in the Endophthalmitis Vitrectomy
Study. Retina 19(2):98–102, 1999. 56. Johnson MW, Doft BH, Kelsey SF, et al: The Endophthalmitis Vitrectomy Study. Relationship between clinical presentation
and microbiologic spectrum. Ophthalmology 104(2):261–272, 1997. 57. Wisniewski SR, Hammer ME, Grizzard WS, et al: An investigation of the hospital charges related to the treatment of endophthalmitis
in the Endophthalmitis Vitrectomy Study. Ophthalmology 104(5):739–745, 1997. 58. Durand M: (Letter to Editor) Microbiologic factors and visual outcome in
the Endophthalmitis Vitrectomy Study. Am J Ophthalmol 124(1):127–130, 1997. 59. Miller JJ, Scott IU, Flynn HW Jr, et al: Endophthalmitis caused by Streptococcus pneumoniae. Am J Ophthalmol 138:231–236, 2004 60. Scott IU, Loo RH, Flynn HW Jr, et al: Endophthalmitis caused by Enterococcus faecalis: antibiotic selection and treatment outcomes. Ophthalmology 110(8):1573–1577, 2003. 61. Benz MS, Scott IU, Flynn HW Jr, et al: Endophthalmitis isolates and antibiotic sensitivities: a 6-year
review of culture-proven cases. Am J Ophthalmol 137:38–42, 2004. 62. Shockley RK, Fishman P, Aziz M, et al: Subconjunctival administration of ceftazidime in pigmented rabbit eyes. Arch Ophthalmol 104:266–268, 1986. 63. Campochiaro PA, Green WR: Toxicity of intravitreous ceftazidime in primate retina. Arch Ophthalmol 110:1625, 1992. 64. Eifrig CWG, Scott IU, Flynn HW Jr, et al: Endophthalmitis caused by Pseudomonas aeruginosa. Ophthalmology 110:1714–1717, 2003. 65. Berrocal AM, Scott IU, Miller D, et al: Endophthalmitis cause by Moraxella osloensis. Graefe's Arch Clin Exp Ophthalmol 240:329–330, 2002. 66. Berrocal AM, Scott IU, Miller D, et al: Endophthalmitis caused by Moraxella species. Am J Ophthalmol 132:788–790, 2001. 67. Scott IU, Matharoo N, Flynn HW Jr, et al: Endophthalmitis caused by Klebsiella species. Am J Ophthalmol 137:525–537, 2004. 68. Davis JL, Koidou-Tsiligianni A, Pflugfelder SC, et al: Coagulase-negative staphylococcal endophthalmitis. Increase in microbial
resistance. Ophthalmology 95:1404–1410, 1988. 69. Cohen SM, Flynn HW Jr, Miller D: Endophthalmitis caused by Serratia marcescens. Ophthalmic Surg Lasers 28:195–200, 1997. 70. Chaudhry NA, Flynn HW Jr, Smiddy WE, et al: Xanthomonas maltophilia endophthalmitis after cataract surgery. Arch Ophthalmol 118:572–575, 2000. 71. Yoder DM, Scott IU, Flynn HW Jr, et al: Endophthalmitis caused by Haemophilus influenzae. Ophthalmology 111:2023–2026, 2004. 72. Oum BS, D'Amico DJ, Wong KW: Intravitreal antibiotic therapy with vancomycin and aminoglycoside: an
experimental study of combination and repetitive injections. Arch Ophthalmol 107:1055, 1989. 73. Olson JC, Flynn HW Jr, Forster RK, et al: Results in the treatment of postoperative endophthalmitis. Ophthalmology 90:692, 1983. 74. Friberg TR: En bloc removal of inflammatory fibrocellular membranes from the iris surface
in endophthalmitis. Arch Ophthalmol 109:736, 1991. 75. Doft BH, Weiskopf J, Nilsson-Ehle I, et al: Amphotericin B clearance in vitrectomized versus nonvitrectomized eyes. Ophthalmology 92:1601, 1985. 76. Campochiaro PA, Conway BP: Aminoglycoside toxicity—a surgery of retinal specialists. Arch Ophthalmol 109:1946, 1991. 77. Campochiaro PA, Lim JL, and the Aminoglycoside Toxicity Study Group: Aminoglycoside toxicity in the treatment of endophthalmitis. Arch Ophthalmol 112:48, 1994. 78. Doft BM, Kelsey SF, Wisniewski SR, and EVS study group. Retinal detachment in the Endophthalmitis Vitrectomy Study. Arch Ophthalmol 118:1661–1665, 2000. 79. Foster RE, Rubsamen PE, Joondeph BC, et al: Concurrent endophthalmitis and retinal detachment. Ophthalmology 101:490, 1994. 80. Pavan PR, Brinser JH: Exogenous bacterial endophthalmitis treated without systemic antibiotics. Am J Ophthalmol 104:121, 1987. 81. Hariprasad SM, Mieler WF, Holz ER: Vitreous penetration of orally administered gatifloxacin in humans. Trans Am Ophthalmol Soc 100:153–159, 2002. 82. Fiscella RG, Lai WW, Buerk B, et al. Aqueous and Vitreous penetration of Linezolid (Zyvox) after oral
administration. Ophthalmology 111:1191–1195, 2004. 83. Barza M. Antibacterial agents in the treatment of ocular infections. Infect Dis Clin North Am 3:533, 1989. 84. Iyer MN, Han DP, Yun HJ, et al: Subconjunctival antibiotics for acute post-cataract extraction endophthalmitis—Is
it necessary? Am J Ophthalmol 137:1120–1121, 2004. 85. Smiddy WE, Smiddy RJ, Ba'arah B, et al: Subconjunctival antibiotics in the treatment of endophthalmitis. Submitted. 86. Das T, Jaleli S, Grothwal V, et al: Intravitreal dexamethasone in exogenous bacterial endophthalmitis: result
of a prospective randomized study. Br J Ophthalmol 83:1050–1055, 1999. 87. Irvine WD, Flynn HW Jr, Miller DA, et al: Endophthalmitis caused by gram-negative organisms. Arch Ophthalmol 110:1450–1444, 1992. 88. Mao LK, Flynn HW Jr, Miller D, et al: Endophthalmitis caused by Staphylococcus aureus. Am J Ophthalmol 116:584, 1993. 89. Koul S, Philipson BT, Philipson A: Visual outcome of endophthalmitis in Sweden. Acta Ophthalmol 67:504, 1989. 90. Jaffe GJ, Abrams GW, Williams GA, et al: Tissue plasminogen activator for postvitrectomy fibrin formation. Ophthlamology 97:184, 1990. 91. Vote BJ, Buttery R, Polkinghorne PJ: Endophthalmitis after intravitreal injection of frozen preprepared tissue
plasminogen activator (tPA) for pneumatic displacement of
submacular hemorrhage. Retina 24:808–809, 2004. 92. Huang S, Brod RD, Flynn HWJr: Management of endophthalmitis while preserving the uninvolved crystalline
lens. Am J Ophthalmol 112:695, 1991. 93. Hopen G, Mondino BJ, Kozy D, et al: Intraocular lenses and experimental bacterial endophthalmitis. Am J Ophthalmol 94:402, 1982. 94. Meisler DM, Palestine AG, Vastine DW, et al: Chronic Propionibacterium endophthalmitis after extracapsular cataract
extraction and intraocular lens implantation. Am J Ophthalmol 102:733, 1986. 95. Scott IU, Lieb DF, Flynn HW Jr: Endophthalmitis caused by Mycobacterium chelonae; selection of antibiotics and outcomes of treatment. Arch Ophthalmol 121:573–576, 2003. 96. Stern WH, Tamura E, Jacobs RA, et al.: Epidemic postsurgical Candida parasilosis endophthalmitis: clinical findings and management of 15 consecutive cases. Ophthalmology 92:170, 1985. 97. Petit TH, Olson RJ, Foos RY, et al: Fungal endophthalmitis following intraocular lens implantation. A surgical
epidemic. Arch Ophthalmol 98:1025, 1980. 98. Scott IU, Cruz-Villegas V, Flynn HW Jr, et al: Delayed-onset, bleb-associated endophthalmitis caused by Lecythophora mutabilis. Am J Ophthalmol 137:583–585, 2004. 99. Hariprasad SM, Mieler WF, Holz ER, et al: Determination of vitreous, aqueous, and plasma concentration of orally
administered voriconazole in humans. Arch Ophthalmol 122:42–47, 2004. 100. Scott IU, Flynn HW Jr, Miller D, et al: Exogenous endophthalmitis caused by amphotericin B-resistant Paecilomyces lilacinus: treatment options and visual outcomes. Arch Ophthalmol 119:916–919, 2001. 101. Gao H, Pennesi ME, Shah K, et al: Intravitreal voriconazole. An electroretinographic and histologic study. Arch Ophthalmol 122:1687–1692, 2004. 102. Gedde SJ, Scott IU, Tabandeh H, et al: Late endophthalmitis associated with glaucoma drainage implants. Ophthalmology 108:1323–1327, 2001. 103. Heilskov T, Joondeph BC, Olsen KR, et al: Late endophthalmitis after transscleral fixation of a posterior chamber
intraocular lens. Arch Ophthalmol 107:1427, 1989. 104. Kangas TA, Greenfield D, Flynn HW Jr, et al: Delayed-onset endophthlamitis associated with conjunctival filtering
blebs. Ophthalmology 104:742–752, 1997. 105. Boldt HC, Pulido JS, Blodi CF, et al: Rural endophthalmitis. Ophthalmology 96:1722, 1989. 106. Vahey JB, Flynn HW Jr: Results in the management of Bacillus endophthalmitis. Ophthalmic Surg and Laser 22:681, 1991. 107. Kervick GN, Flynn HW Jr, Alfonso E, et al: Antibiotic therapy for Bacillus species infections. Am J Ophthalmol 110:683, 1990. 108. Hemady R, Zaltas M, Paton B, et al: Bacillus-induced endophthalmitis: new series of 10 cases and review
of the literature. Br J Ophthalmol 74:26, 1990. 109. Foster RE, Martinez JA, Murray TG, et al: Useful visual outcomes after treatment of Bacillus cereus endophthalmitis. Ophthalmology 103:390–397, 1996. 110. Mieler WF, Ellis MK, Williams DF, et al: Retained intraocular foreign bodies and endophthalmitis. Ophthalmology 1990:97, 1532. 111. Lieb DF, Scott IU, Flynn HW Jr, et al: Open globe injuries with positive intraocular cultures: factors influencing
final visual acuity outcomes. Ophthalmology 110:1560–1566, 2003. 112. Feist RM, Lim JI, Joondeph BC, et al: Penetrating ocular injury from contaminated eating utensils. Arch Ophthalmol 109–163, 1991. 113. Reynolds DS, Flynn HW Jr: Endophthalmitis after penetrating ocular trauma. Current Opin in Ophthalmol 8:32–38, 1997. 114. Flynn HW Jr, Essman TF, Brod RD: Endogenous fungal endophthalmitis. In: Saer JB (ed.). Vitreous Retinal and Uveitis Update. Hague, Netherlands: Kugler Publishers, 1998:297–305. 115. Tornambe PE, Hilton GF, and the Retinal Detachment Study Group. Pneumatic retinopexy. A multicenter randomized control clinical trial comparing
pneumatic retinopexy with scleral buckling. Ophthalmology 96:772–784, 1989. 116. Eckardt C. Staphylococcus epidermidis endophthalmitis after pneumatic retinopexy. Am J Ophthalmol 103:720–721, 1987. 117. Ho PC, McNeel JW. Bacterial endophthalmitis after retinal surgery. Retina ; 3:99, 1983. 118. Shrader SK, Band JD, Lauter CB, et al: The clinical spectrum of endophthalmitis: incidence, predisposing factors, and
features influencing outcome. J Infect Dis 162:115, 1990. 119. Sherwood DR, Fich WJ, Jacob JS, et al: Bacterial contamination of intraocular and extraocular fluids during extracapsular
cataract extraction. Eye 3:308, 1989. 120. Speaker MG, Milch FA, Shah MK, et al: Role of external bacterial flora in the pathogenesis of acute postoperative
endophthalmitis. Ophthalmology 98:639, 1991. 121. Menikoff JA, Speaker MG, Marmor M, et al: A case-control study of risk factors for postoperative endophthalmitis. Ophthalmology 98:1761, 1991. 122. Isenberg SL, Apt L, Yoshmori R, et al: Chemical preparation of the eye in ophthalmic surgery: IV comparison of
povidone-iodine on the conjunctiva with a prophylactic antibiotic. Arch Ophthalmol 103:1340, 1985. 123. Whitney CR, Anderson RP, Allansmith MR: Preoperatively administered antibiotics: their effect on bacterial counts
of the eyelids. Arch Ophthalmol 87:155, 1972. 124. Apt L, Isenberg S, Yoshimori R, et al: Chemical preparation of the eye in ophthalmic surgery: III effect of povidone-iodine
on the conjunctiva. Arch Ophthalmol 102:728, 1984. 125. Speaker MG, Menikoff JA: Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology 98:1769, 1991. 126. Alfonso EC, Flynn HW Jr. Controversies in endophthlamitis prevention. The risk for emerging resistance
to vancomycin. Arch Ophthalmol 113:1369–1370, 1995. 127. Centers for Disease Control and Prevention: Preventing the spread of vancomycin-resistance. Report from the
Hospital Infection Control Practices Advisory Committee. 59 Federal Register 25:757, 1994. 128. Axer-Siegel R, Stiebel-Kalish H, Rosenblatt I, et al: Cystoid macular edema after cataract surgery with intraocular vancomycin [see comment]. [Clinical Trial. Journal Article. Randomized
Controlled Trial]. Ophthalmology . 106:1660–1664, 1999. 129. Ciulla TA, Starr MB, Masket S: Bacterial endophthalmitis prophylaxis for cataract surgery. An evidence-based
update. Ophthalmology 109:13–26, 2002. |