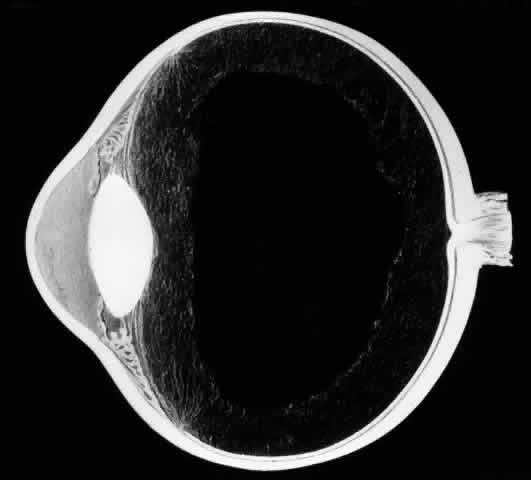
It is important to distinguish between a giant retinal tear and a retinal dialysis greater than 90° because their surgical management is different. Most giant retinal tears occur at the posterior border of the vitreous base or along the posterior margin of lattice degeneration. A dialysis occurs along the ora serrata as a separation of the neurosensory retina from the nonpigmented ciliary epithelium. Dialyses are three times more common than giant tears and constitute approximately 10% of rhegmatogenous retinal detachments.5,6 Superior nasal dialyses may result from a traumatic avulsion of the vitreous base. Spontaneous dialyses are often bilateral and usually involve the inferotemporal quadrant. Dialyses produce a retinal detachment that develops slowly and is often characterized by concentric pigmented demarcation lines. Vitreous detachment is rare in eyes with dialyses.
The vitreous is almost always attached to the posterior edge of the dialysis, therefore inversion of the retina occurs infrequently.7,8 Scleral buckling of retinal dialyses is usually successful; anatomic reattachment rates of greater than 95% are common.5
PREOPERATIVE EXAMINATION
Indirect Ophthalmoscopy
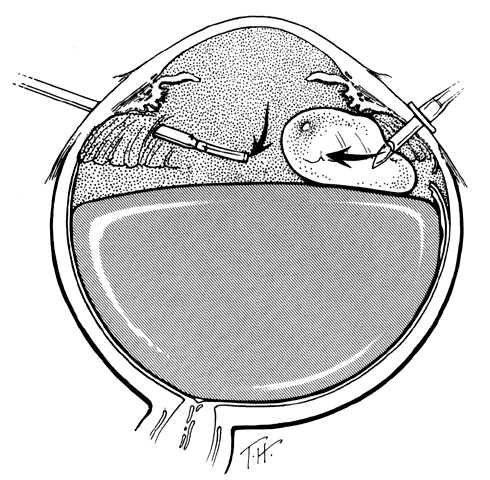
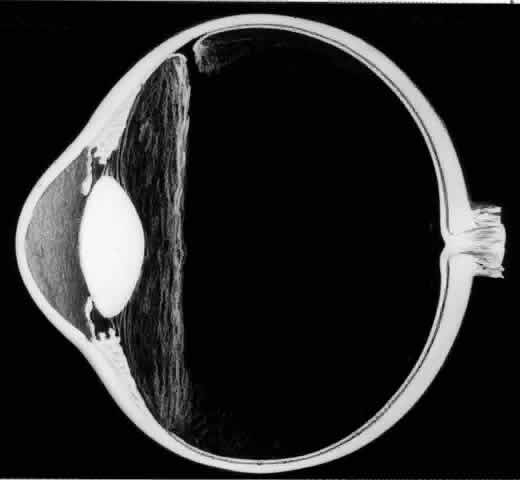
The outer surface of the inverted retinal flap (Fig. 1) should be examined for signs of epiretinal tissue proliferation, which may produce radial or star-shaped folds in the flap. Vitreous base traction produces detachment of the anterior edge of the giant tear and the pars plana epithelium anterior to it in 82% of giant retinal tears.9 It is important to identify and treat detachment of the pars plana epithelium that extends beyond the ends of the giant tear because leakage of subretinal fluid beneath it can be a potential source of postoperative posterior retinal detachment.
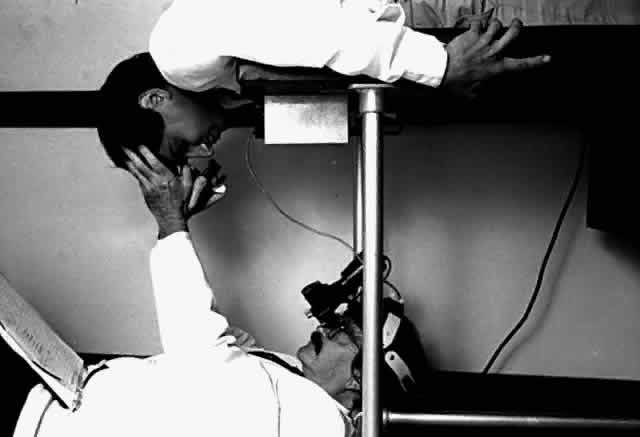
Indirect ophthalmoscopy of the patient in the prone position is helpful to determine the mobility of the inverted retinal flap and the extent to which it will unfold. During indirect ophthalmoscopy, the patient lies prone with his or her head extended over one end of the examining table, while the examiner sits cross-legged or lies on the floor (Fig. 2). If the flap unfolds poorly, either epiretinal tissue formation involving the posterior retinal flap or early proliferative vitreoretinopathy should be suspected. Preretinal organization may be present when the posterior edge of the flap is rolled in a scroll-like fashion, creating a purse-string contracture that prevents complete unfolding of the flap.
Retinal breaks have been reported to occur in 59% of the fellow eyes of nontraumatic giant retinal tears, which is why indirect ophthalmoscopy of the fellow eye is extremely important.3
Biomicroscopy
Biomicroscopy of the vitreous is performed with the Goldmann three-mirror contact lens. Biomicroscopy reveals extensive liquefaction of the central and posterior vitreous gel and marked condensation of the anterior vitreous and vitreous base. In some eyes, a membrane attached to the anterior edge of the giant retinal tear extends across the vitreous cavity, attaching to the retina in the region of the posterior border of the vitreous base. Condensation and contraction of the vitreous base pulls the anterior edge of the giant tear toward the lens, resulting in detachment of the ora serrata and pars plana epithelium.
SURGICAL MANAGEMENT
Sclerotomies
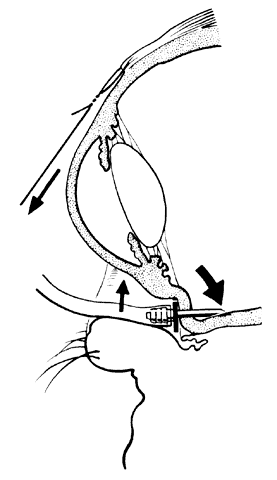
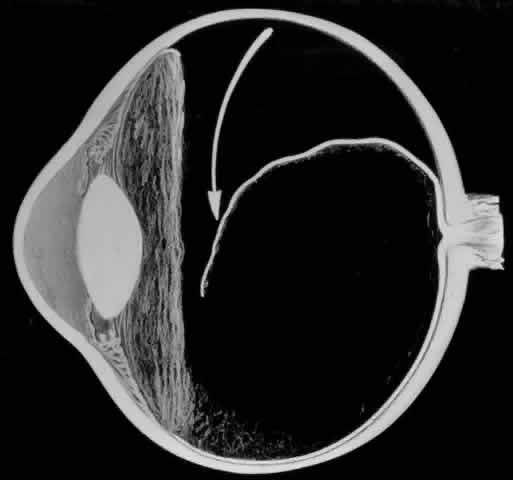
To perform vitrectomy involving the inferior vitreous base, it may be necessary to rotate the globe so far inferiorly that the outer part of the infusion cannula is forced against the lower eyelid. This may cause the inner tip of the cannula to come in contact with, and thus damage, the peripheral retina (Fig. 3).10 To avoid this complication, the site of the infusion cannula should be located near the horizontal meridian along the inferior margin of the lateral rectus muscle. A gentian violet pen is used to make a mark on the sclera 3.5 mm posterior to the limbus. A mattress suture of 6-0 polyglactin with a spatula needle is preplaced with the arms of the suture parallel to the limbus and 1 mm anterior and posterior to the mark. As Glaser11 pointed out, a stream of infusion fluid can disrupt a perfluoro-n-octane bubble into smaller bubbles, which is why a short infusion cannula is used. To facilitate insertion of the cannula through the condensed vitreous base, thus avoiding detaching the pars plana epithelium, the slit-like opening made by the microvitreal knife is enlarged with a stiletto rotated 180°. After its insertion, the short cannula is depressed toward the center of the globe to check that the tip has entered the vitreous cavity and is not beneath a detached pars plana epithelium.
Sclerotomies are made in the 2:30 and 9:30 meridians for the vitrectomy probe and the endoillumination probe, respectively. Passing the flat tip of the endoilluminator probe through the condensed vitreous base can produce a detachment of the pars plana epithelium. Therefore, the probe is slowly inserted into the sclerotomy with a rotating motion, and the tip is inspected to ensure that the pars plana epithelium or ora serrata are not being pushed in front of the probe as it enters the vitreous cavity.
Lensectomy
Recent reports indicate that scleral buckling may not be necessary in the management of a giant retinal tear, provided that traction is relieved as a result of a thorough vitrectomy involving the vitreous base.12,13 Kreiger13 believes that a lensectomy is necessary for optimal removal of the basal vitreous, and I am inclined to agree. When no nuclear sclerosis is present, lensectomy is performed with the vitrectomy probe. Phacoemulsification is used for a mildly sclerotic lens; a very hard or brunescent lens is removed intracapsularly through a corneal incision, which is closed with multiple interrupted sutures. An argument against lensectomy is that the average age of a patient presenting with a giant retinal tear is 32 years, an age at which the lens is clear. Therefore, some advocate preservation of the phakic status in these relatively young patients. Postoperative tamponade with a longacting gas may produce or accelerate cataractous changes that reduce visual acuity or hinder postoperative photocoagulation and necessitate cataract extraction. If the surgeon elects to preserve the lens, it is advisable to use perfluoroethane (C2F6) gas, which has a shorter half-life than perfluoropropane (C3F8). Additional studies will help to establish whether or not lensectomy is necessary.
Optimal Visualization During Surgery
A wide-angle fungus-viewing system such as the Advanced Visual Instrument (AVI) system is of great value during vitrectomy involving the vitreous base and during the unfolding of the inverted retinal flap. This system uses an image inverter attached to the operating microscope and a set of two contact lenses with 68° and 130° viewing angles. The 130° lens provides a panoramic view of the fundus as far anterior as the ora serrata.14
Anterior Vitrectomy
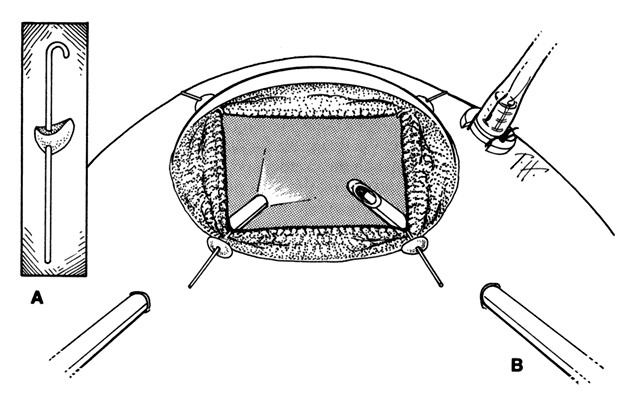
To promote maximal mydriasis, 1 mL of 1:1000 epinephrine is added to 500 mL of the balanced salt infusion solution. If a small pupil hinders visualization, four flexible nylon iris retractors are used for temporary pupillary dilation.15 Each plastic retractor measures 6 mm in length and has a 1-mm hook at one end and a small polymeric silicone (Silastic) disc to hold it in place. The iris retractors are inserted through the limbus in the 1:30, 4:30, 7:30, and 10:30 meridians (Fig. 4). Anterior vitrectomy is performed to remove the condensed anterior vitreous gel. It is important to remove the vitreous gel attached to the anterior edge of the giant retinal tear, or else this gel may be pushed posteriorly during air infusion and interfere with the unfolding of the flap or cause the posterior edge of the giant tear to slip posteriorly (Fig. 5).13 It is important to identify and remove the membrane that is attached to the anterior flap of the giant tear and extends across the vitreous cavity to the vitreous base opposite the giant tear. This membrane may be visualized best with illumination slightly eccentric to the tip of the vitrectomy probe. Removal of this membrane will decrease the incidence of retinal tears that have been observed to develop opposite the giant retinal tear.
Vitrectomy Involving the Vitreous Base
A thorough vitrectomy of the vitreous base around its entire circumference is important in the management of a giant tear without scleral buckling. Because of the strong adhesion between the peripheral retina and vitreous base, vitrectomy involving the vitreous base carries a considerable risk of causing iatrogenic retinal breaks, especially if the vitrectomy probe does not cut efficiently. Scleral depression by the assistant during the procedure allows enhanced visualization of the vitreous base. After the vitrectomy is completed, the entire fundus periphery is examined with the use of indirect ophthalmoscopy and scleral depression to determine whether the giant tear has become enlarged or to detect iatrogenic peripheral retinal breaks.
UNFOLDING THE POSTERIOR RETINAL FLAP
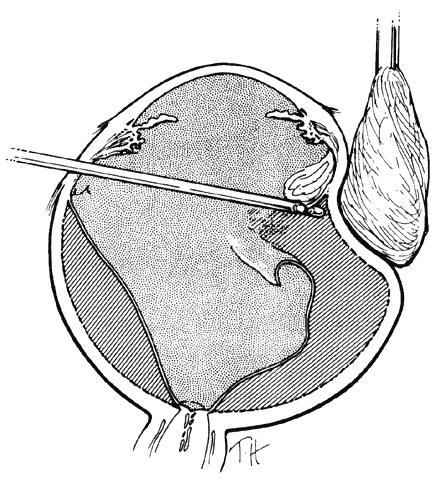
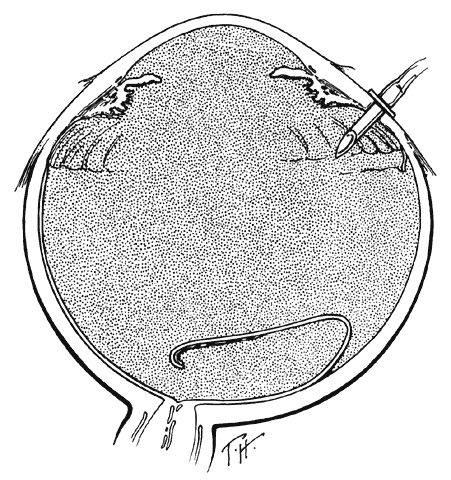
One of the most challenging problems in the management of giant retinal tears has been unfolding the inverted posterior retinal flap (Fig. 6). Schepens16 demonstrated that gravity can be used to unfold the flap by placing the patient in the prone position. This led to the development of a multipositional giant tear operating table used to rotate the patient to the prone position during surgery.17,18 Norton and associates19 and Michels20 reported the use of air injection into the vitreous cavity to unfold and tamponade the posterior retinal flap. One of the most important contributions in the management of giant retinal tears with an inverted retinal flap was made by Chang and colleagues,21–24 who demonstrated the value of liquid perfluorocarbons in unfolding the inverted retinal flap. Because this procedure is performed with the patient in the supine position, unfolding of an inverted retinal flap is accomplished more easily and with fewer complications.
Perfluoro-n-Octane
Perfluoro-n-octane is an ideal heavier-than-water liquid for unfolding the inverted retinal flap of a giant retinal tear. Its specific gravity of 1.76 compared with 1.03 of aqueous causes perfluoro-noctane to gravitate posteriorly, settling on the surface of the posterior retina and optic disc. The surface tension of 16.98 accounts for the tendency of injected perfluoro-n-octane to remain in one globule as the globule increases in size to unfold and tamponade the posterior retinal flap of the giant retinal tear.
Perfluoro-n-octane (C8F18) is an inert perfluorocarbon: there are no known biologic enzymes that can metabolize its carbon-fluoride bonds. It is optically clear, permitting excellent visualization of the retina; however, the edge of the bubble of perfluoro-n-octane is visible within the eye because perfluoro-n-octane has a different refractive index (1.27) than aqueous (1.33) or balanced salt infusion. Perfluoro-n-octane has a vapor pressure of 52 mmHg at 37°C, which tends to volatilize it during fluid-gas exchange. Its low viscosity (0.69 centistokes) and its surface tension account for the ease with which the gas can be removed from the eye.
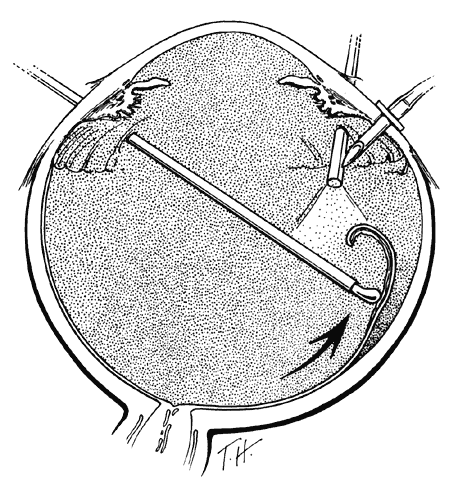
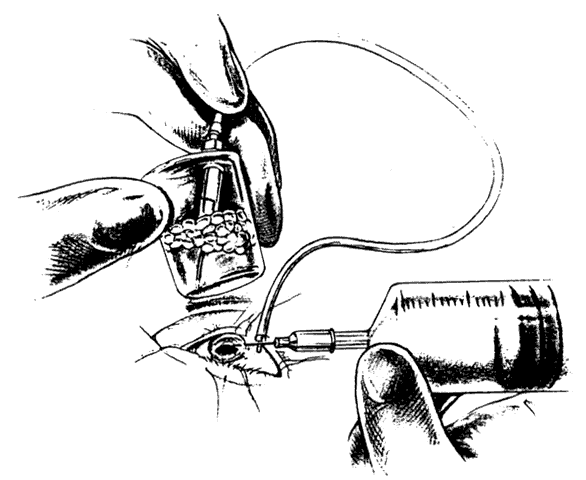
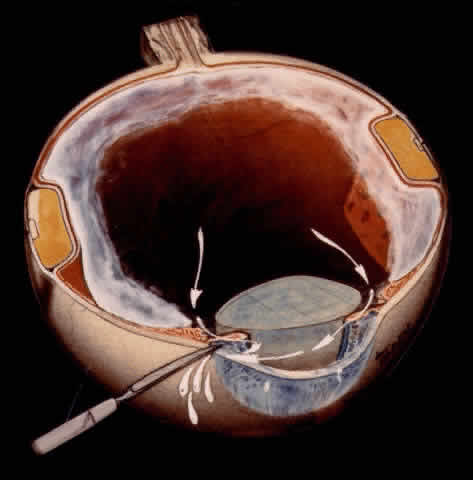
TECHNIQUE. With the tip of the vitrectomy probe or a retinal manipulator (i.e., an angulated probe with a ball-like tip), the inverted retinal flap is partially unfolded to expose the optic disc and posterior pole (Fig. 7). Perfluoro-n-octane is injected slowly through a blunt-tipped 25-gauge needle positioned 1 to 2 mm over the optic disc (Fig. 8), creating a globule. As the globule expands toward the fundus periphery, it smoothly unfolds the posterior retinal flap. To prevent the formation of multiple bubbles of perfluoro-n-octane, the tip of the injection needle must be kept within the globule during injection.
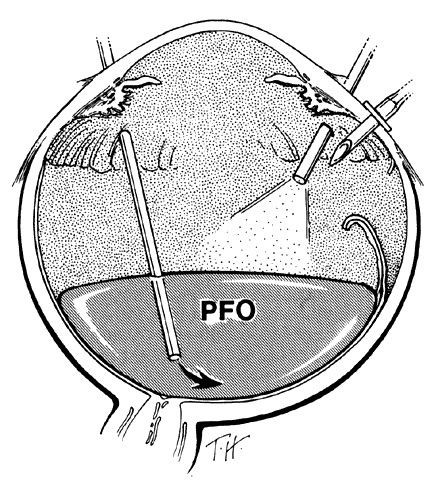
If epiretinal membranes are causing the posterior edge of the flap to roll or curl, they can be removed as follows. An injection of 0.8 to 1 mL perfluoro-n-octane is used as a means of partially unfolding the flap and steadying it to enable bimanual dissection of the epiretinal membranes. After the membranes are removed, additional perfluoro-n-octane is injected to bring the level of the perfluoro-n-octane to the posterior edge of the giant tear (Fig. 9).
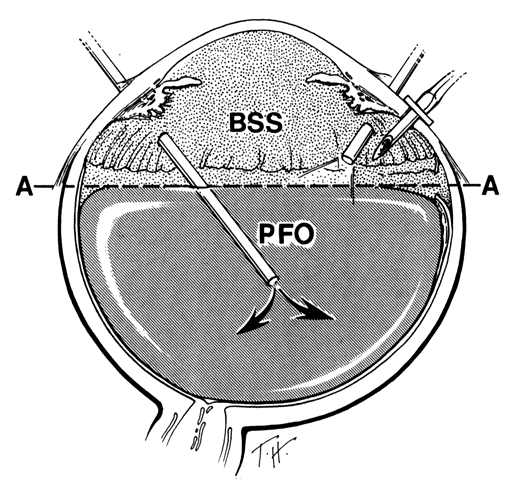
Anterior Fluid-Air Exchange. Using the cannulated extrusion needle with a suction pressure of approximately 70 mmHg, the fluid anterior to the perfluoro-n-octane is removed by fluid-air exchange (Fig. 10).25 Fluid along or under the posterior edge of the giant tear promotes posterior slippage of the posterior edge of the giant tear. Therefore, it is extremely important to remove as much of this fluid as possible (Fig. 11). Slight slippage of the retinal flap can be corrected by massaging the edge of the retina anteriorly with the retinal manipulator. If this fails, the posterior edge of the flap is dragged anteriorly with minimal suction through the soft silicone tip of the cannulated extrusion needle, which is applied to the displaced posterior edge of the giant retinal tear. An effort is made to flatten the posterior edge of the giant tear completely in order to prevent perfluoro-n-octane from passing beneath it. If any perfluoro-n-octane migrates subretinally beneath the flap, it is removed with the cannulated extrusion needle or via retinotomy.
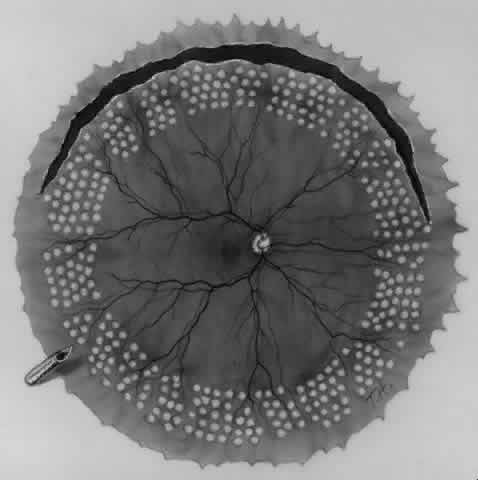
Endolaser. Eight to 10 rows of endophotocoagulation are applied to the anterior third of the posterior retinal flap (Fig. 12). Five rows of endophotocoagulation are placed from the ora serrata to the equator in the fundus periphery not involved in the giant tear so that treatment extends around the entire periphery of the fundus.
Removal of Perfluoro-n-Octane. Perfluoro-noctane is removed with the cannulated extrusion needle or double-barreled vented needle. As the air meniscus approaches the retina, the layer of perfluoro-n-octane becomes flattened, making the small residual amount difficult to visualize. Approximately 0.5 to 0.8 mL of balanced salt solution is then injected into the eye to cause the perfluoro-n-octane to form small droplets in the saline phase, which can be seen and removed more easily.
Air-Gas Exchange. To allow air to be vented from the eye during an air-gas exchange, the infusion tubing is clamped and disconnected from the three-way stop cock. To produce a slow venting of air through a smaller diameter than that of the infusion tubing, and thus prevent a sudden decrease in intraocular pressure, a 25-gauge needle is attached at the end of the tubing using a female adapter. Air is exchanged for a 15% mixture of C3F8 and air that is injected from a 50-cc syringe with a 30-gauge needle inserted through the limbus in aphakic eyes and through the pars plana in phakic and pseudophakic eyes. After the needle has entered the eye, the clamp on the infusion cannula is removed and 40-cc of the gas mixture is lavaged through the eye. Air and gas is vented through the infusion tubing and needle that has been placed in balanced salt solution in a beaker. The continuous flow of bubbles in the beaker indicates a continuous lavage (Fig. 13).
Air-Silicone-Fluorosilicone Oil Exchange. For infants and children in whom postoperative prone positioning is not possible and in cases of severe proliferative vitreoretinopathy, an air-oil exchange is performed with a silicone-fluorosilicone oil (SiFO) copolymer developed by Refojo, Tolentino, and associates.26 This copolymer has a viscosity of 180 centistokes, a specific gravity of 1.15 g/cm3, and a refractive index of 1.38. SiFO seems preferable to silicone oil for postoperative retinal tamponade because its higher specific gravity causes it to gravitate posteriorly against the retina, whereas silicone oil tends to float anteriorly, thus producing a higher incidence of corneal complications and therefore less tamponading of the inferior retina. SiFO is injected to fill the vitreous cavity to a level slightly posterior to the plane of the pupil. The oil is removed and replaced with balanced salt solution 3 to 6 months postoperatively or earlier if marked emulsification of the oil occurs.