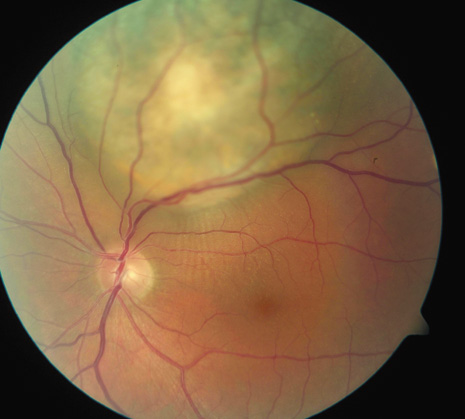
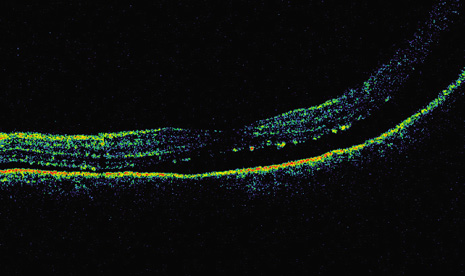
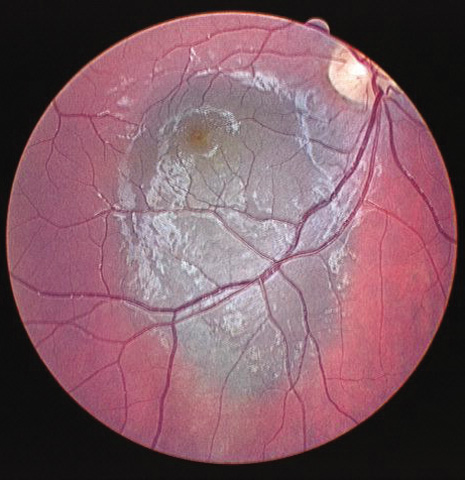
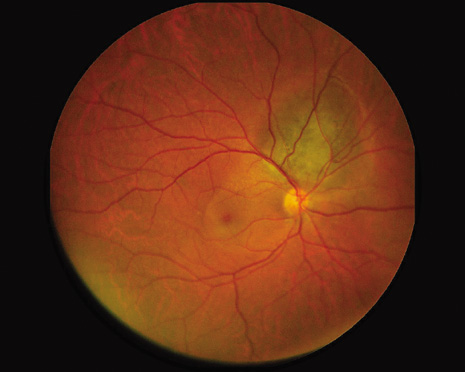
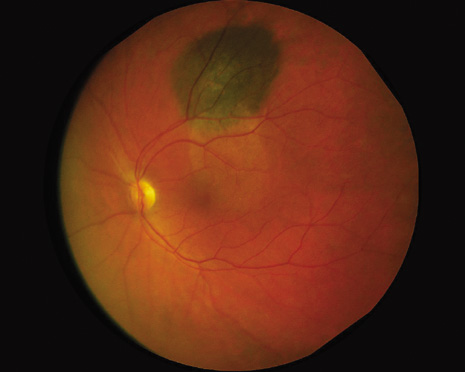
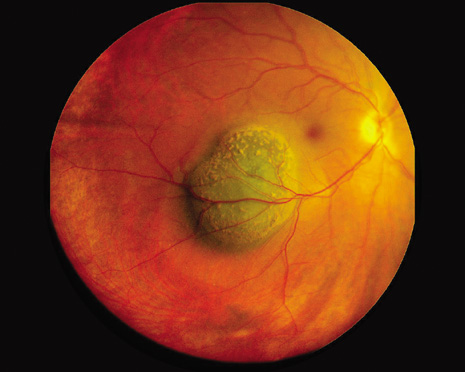
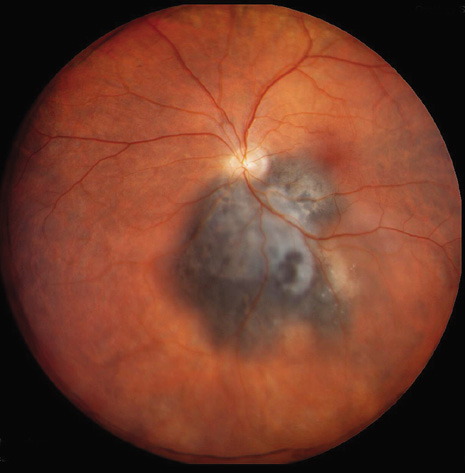
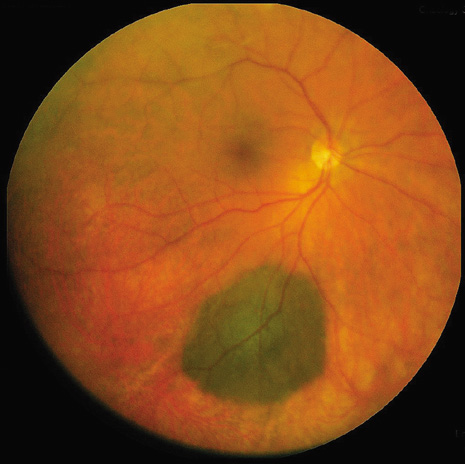
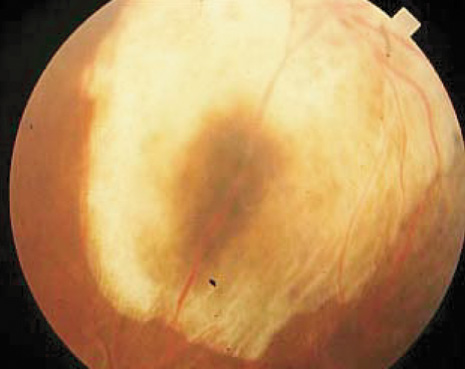
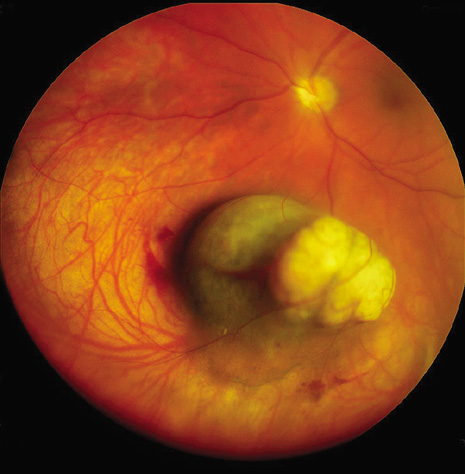
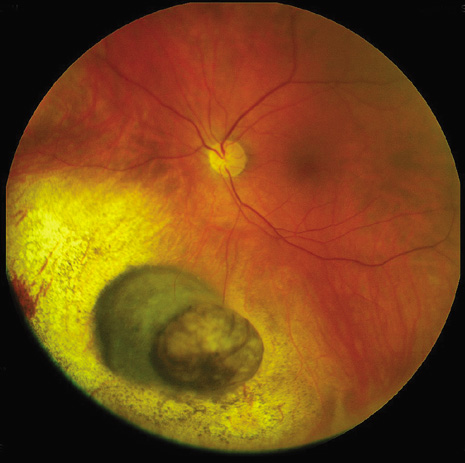
1. Robertson DM: Changing concepts in the management of choroidal melanoma. Am J Ophthalmol 136:161, 2003 2. Shields CL, Shields JA, Gunduz K, et al: Radiation therapy for uveal malignant melanoma. Ophthalmic Surg Lasers 29:397, 1998 3. Shields JA, Shields CL: Surgical approach to lamellar sclerouvectomy for posterior uveal melanomas. Ophthalmic Surg 19:774, 1988 4. Shields CL, Shields JA: Recent developments in the management of choroidal melanoma. Curr Opin Ophthalmol 39:351, 2004 5. Zimmerman LE, McLean IW: An evaluation of enucleation in the management of uveal melanomas. Am J Ophthalmol 87:741, 1979 6. Zimmerman LE, McLean IW, Foster WD: Does enucleation of the eye containing a malignant melanoma prevent or
accelerate the dissemination of tumour cells? Br J Ophthalmol 62:420, 1978 7. Manschot WA, Van Peperzeel HA: Choroidal melanoma: Enucleation or observation? A new approach. Arch Ophthalmol 98:71, 1980 8. Seigel D, Myers M, Ferris F, Steinhorn SC: Survival rates after enucleation of eyes with malignant melanoma. Am J Ophthalmol 87:751, 1979 9. Straatsma BR, Fine SL, Earle JD: The Collaborative Ocular Melanoma Study Research Group. Enucleation versus
plaque irradiation for choroidal melanoma. Ophthalmology 95:1000, 1988 10. Collaborative Ocular Melanoma Study Group: Trends in size and treatment
of recently diagnosed choroidal melanoma, 1987–1997: Findings from
patients examined at collaborative ocular melanoma study (COMS) centers. Arch Ophthalmol 121:1156, 2003 11. Shields CL, Cater JC, Shields JA, et al: Combination of clinical factors predictive of growth of small choroidal
melanocytic tumors. Arch Ophthalmol 118:360, 2000 12. Shields CL, Shields JA, Kiratli H, et al: Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology 102:1351, 1995 13. Sumich P, Mitchell P, Wang JJ: Choroidal nevi in a white population: The Blue Mountains Eye Study. Arch Ophthalmol 116:645, 1998 14. Ganley JP, Comstick GW: Benign nevi and malignant melanomas of the choroids. Am J Ophthalmol 76:19, 1973 15. Shields CL, Shields JA: Clinical features of small choroidal melanoma. Curr Opin Ophthalmol 13:135, 2002 16. Shields CL, Demirci H, Materin MA, et al: Clinical factors in the identification of small choroidal melanoma. Can J Ophthalmol, 39:351, 2004 17. Straatsma BR, Diener-West M, Caldwell R, Engstrom RE: Collaborative Ocular Melanoma Study Group. Mortality after deferral of
treatment or no treatment for choroidal melanoma. Am J Ophthalmol 136:47, 2003 18. Foulds WS, Damato BE: Low energy long-exposure laser therapy in the management of choroidal melanoma. Graefes Arch Clin Exp Ophthalmol 224:26, 1986 19. Shields JA, Glazer LC, Mieler WF, Shields CL: Comparison of xenon arc and argon laser photocoagulation in the treatment
of choroidal melanomas. Am J Ophthalmol 109:647, 1990 20. Barbazetto IA, Lee TC, Rollins IS, et al: Treatment of choroidal melanoma using photodynamic therapy. Am J Ophthalmol 135:898, 2003 21. Oosterhuis JA, Journee-de Korver HG, Kakebeeke-Kemme HM, Bleeker JC: Transpupillary thermotherapy in choroidal melanomas. Arch Ophthalmol 113:315, 1995 22. Shields CL, Shields JA, Cater J, et al: Transpupillary thermotherapy for choroidal melanoma. Tumor control and
visual outcome in 100 consecutive cases. Ophthalmology 105:581, 1998 23. Shields CL, Shields JA, DePotter P, Kheterpel S: Transpupillary thermotherapy in the management of choroidal melanoma. Ophthalmology 103:1642, 1996 24. Shields CL, Shields JA, Perez N, et al: Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive
cases: Outcomes and limitations. Ophthalmology 109:225, 2002 25. Singh AD, Eagle RC Jr, Shields CL, Shields JA: Enucleation following transpupillary thermotherapy of choroidal melanoma: Clinicopathologic
correlations. Arch Ophthalmol 121:397, 2003 26. Zaldivar RA, Aaberg TM, Sternberg P Jr, et al: Clinicopathologic findings in choroidal melanomas after failed transpupillary
thermotherapy. Am J Ophthalmol 135:657, 2003 27. Shields CL, Cater J, Shields JA, et al: Combined plaque radiotherapy and transpupillary thermotherapy for choroidal
melanoma in 270 consecutive patients. Arch Ophthalmol 120:933, 2002 28. Rem AI, Oosterhuis JA, Journee-de Korver HG, et al: Transscleral thermotherapy: Short- and long-term effects of transscleral
conductive heating in rabbit eyes. Arch Ophthalmol 121:510, 2003 29. Collaborative Ocular Melanoma Study (COMS) randomized trial of
I-125 brachytherapy for choroidal melanoma. III. Initial mortality findings. Ophthalmology 108:348, 2001 30. The Collaborative Ocular Melanoma Study Group: The COMS randomized trial
of Iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality
findings. Arch Ophthalmol 119:969, 2001 31. DePotter , P, Shields CL, Shields JA, et al: The impact of enucleation versus plaque radiotherapy in the management
of juxtapapillary choroidal melanoma on patient survival. Br J Ophthalmol 78:109, 1994 32. Gunduz K, Shields CL, Shields JA, et al: Plaque radiotherapy for management of ciliary body and choroidal melanoma
with extrascleral extension. Am J Ophthalmol 130:97, 2000 33. Gunduz K, Shields CL, Shields JA, et al: Plaque radiotherapy of uveal melanoma with predominant ciliary body involvement. Arch Ophthalmol 117:170, 1999 34. Shields CL, Naseripour M, Cater J, et al: Plaque radiotherapy for large posterior uveal melanoma (>8 mm in
thickness) in 354 consecutive patients. Ophthalmology 109:1838, 2002 35. Shields CL, Shields JA, Cater J, et al: Plaque radiotherapy for uveal melanoma. Long-term visual outcome in 1106 patients. Arch Ophthalmol 118:1219, 2000 36. Shields JA, Augsberger JJ, Brady LW, Day J: Cobalt plaque therapy of posterior uveal melanomas. Ophthalmology 89:1201, 1982 37. Shields JA, Shields CL, Donoso LA: Management of posterior uveal melanoma. Surv Ophthalmol 36:161, 1991 38. Shields CL, Naseripour M, Shields JA, et al: Custom-designed plaque radiotherapy for nonresectable iris melanoma in 38 patients: Tumor
control and ocular complications. Am J Ophthalmol 135:648, 2003 39. Char DH, Kroll SM, Castro JK: Ten-year follow-up of Helium ion therapy of uveal melanoma. Am J Ophthalmol 125:81, 1998 40. Gragoudas ES, Goitein M, Verhey L, et al: Proton beam irradiation. An alternative to enucleation for intraocular
melanomas. Ophthalmology 89:571, 1980 41. Gragoudas ES, Lane AM, Munzenrider J, et al: Long-term risk of local failure after proton therapy for choroidal/ciliary
body melanoma. Trans Am Ophthalmol Soc 100:43; 2002 42. Seddon JM, Gragoudas ES, Albert DM, et al: Comparison of survival rates for patients with uveal melanoma after treatment
with proton beam irradiation or enucleation. Am J Ophthalmol 99:282, 1985 43. Gunduz K, Shields CL, Shields JA, et al: Radiation complications and tumor control after plaque radiotherapy of
choroidal melanoma with macular involvement. Am J Ophthalmol 127:579, 1999 44. Shields CL, Cater J, Shields JA, et al: Combined plaque radiotherapy and transpupillary thermotherapy for choroidal
melanoma: tumor control and treatment complications in 270 consecutive
patients. Arch Ophthalmol 120:933, 2002 45. Shields CL, Shields JA, Karlsson U, et al: Reasons for enucleation after plaque radiotherapy for posterior uveal melanoma. Ophthalmology 96:919, 1989 46. Shields CL, Shields JA, Karlsson U, et al: Enucleation following plaque radiotherapy: For posterior uveal melanoma. Histopathologic
findings. Ophthalmology 97:1665, 1990 47. Jampol LM, Moy CS, Murray TG, et al: The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. IV. Local
treatment failure and enucleation in the first 5 years
after brachytherapy. Ophthalmology 109:2197, 2002 48. The Collaborative Ocular Melanoma Study Group: The collaborative ocular
melanoma study (COMS) randomized trial of pre-enucleation radiation
of large choroidal melanoma II: Initial mortality findings. Am J Ophthalmol 126:779, 1998 49. The Collaborative Ocular Melanoma Study Group: Factors predictive of growth
and treatment of small choroidal melanoma. Arch Ophthalmol 115:1537, 1997 50. Benson WE: The COMS: Why was it not stopped sooner? Arch Ophthalmol 120:672, 2002 51. Shields JA, Shields CL, Shah P, Sivalingam V: Partial lamellar sclerouvectomy for ciliary body and choroidal tumors. Ophthalmology 98:971, 1991 52. Foulds WS, Damato BE: Alternative to enucleation in the management of choroidal melanoma. Aust NZ J Ophthalmol 14:19, 1986 53. Shields JA, Shields CL, DePotter P: Local resection of posterior uveal tumors. In Update on Malignant Ocular
Tumors. Int Ophthalmol Clin 33:137, 1993 54. Wilson RS, Fraunfelder FT: “No touch” cryosurgical enucleation: A minimal trauma technique
for eyes harboring intraocular malignancy. Ophthalmology 85:1170, 1978 55. Dutton JJ: Coralline hydroxyapatite as an ocular implant. Ophthalmology 98:370, 1991 56. Shields CL, Shields JA, De Potter P: Hydroxyapatite orbital implant after enucleation. Experience with 100 consecutive
cases. Arch Ophthalmol 110:333, 1992 57. Shields CL, Shields JA, DePotter P: Hydroxyapatite orbital implant after enucleation for intraocular tumors. In
Update on Malignant Ocular Tumors. Int Ophthalmol Clin 33:83, 1993 58. Char DH, Phillips TL, Andejeski Y, et al: Failure of pre-enucleation radiation to decrease uveal melanoma mortality. Am J Ophthalmol 106:21, 1988 59. Shields JA, Shields CL, Suvarnamani C, et al: Orbital exenteration with eyelid sparing: indications, technique and results. Ophthalmic Surg 22:292, 1991 60. Shields JA, Shields CL, Demirci H, et al: Experience with eyelid-sparing orbital exenteration. Ophthal Plast Reconstr Surg 17:355, 2001 61. Shields JA: Future management of posterior uveal melanoma. Ophthal Pract 19:4, 2001 62. Aoyama T, Mastrangelo MJ, Berd D, et al: Protracted survival following resection of metastatic uveal melanoma. Cancer 89:1561, 2000 63. Mavligit GM, Charnsangevej C, Carrasco H, et al: Regression of ocular melanoma metastatic to the liver after hepatic arterial
chemoembolization with cisplatin and polyvinyl sponge. JAMA 260:974, 1988 64. Shields JA: Counseling the patient with a posterior uveal melanoma. Am J Ophthalmol 106:88, 1988 65. Shields JA, Shields CL: The management of posterior uveal melanoma. In Intraocular Tumors. A Text and Atlas. Philadelphia: Saunders, 1992:191 |