DIABETIC RETINOPATHY
Diabetic retinopathy is the leading cause of blindness in people 20 to 55 years of age. The most common cause of decreased vision in patients with diabetes is macular edema. Over the past 2 decades, multicenter randomized clinical trials evaluating laser treatment in the setting of diabetic retinopathy have been undertaken. These trials and the practical application of their recommendations are discussed in this chapter.
In 1976, the National Eye Institute completed the Diabetic Retinopathy Study (DRS), a collaborative study to evaluate the effectiveness of scatter PRP for proliferative diabetic retinopathy (PDR).23 More than 1700 patients with advanced retinopathy were enrolled in the study. One eye was arbitrarily assigned to treatment using either xenon arc or argon laser photocoagulation while the other eye was observed. This study showed that both argon and xenon scatter photocoagulation reduced the risk of severe vision loss by 50%.24,25 In this study, “severe vision loss” was defined as visual acuity worse than 5/200 at two consecutive follow-up visits 4 months apart. Because of fewer harmful side effects compared with those associated with xenon, argon is the preferred treatment modality.
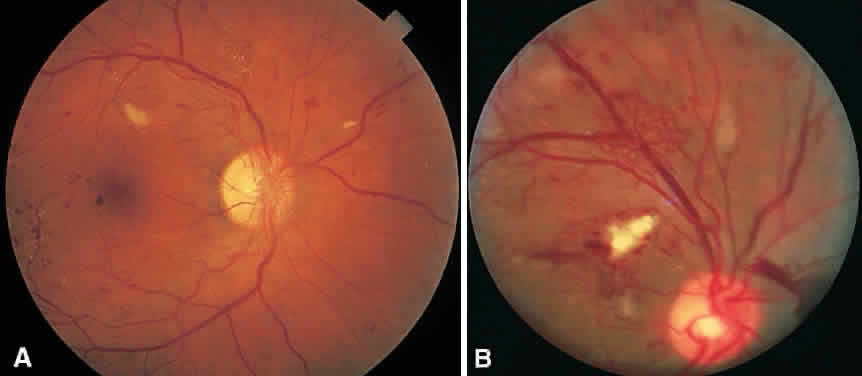
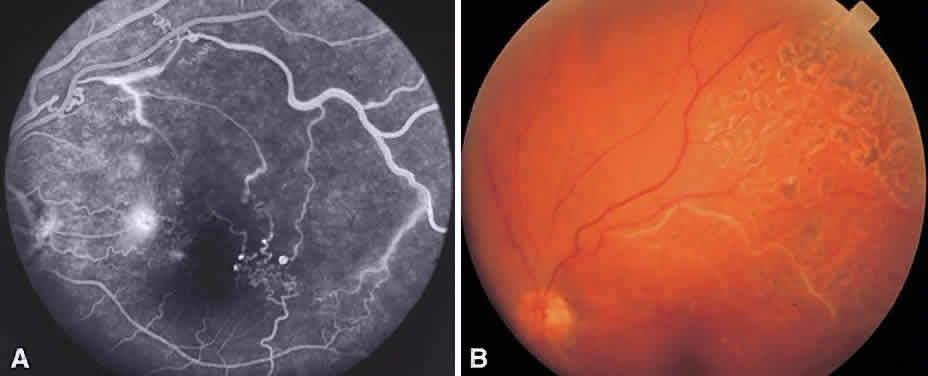
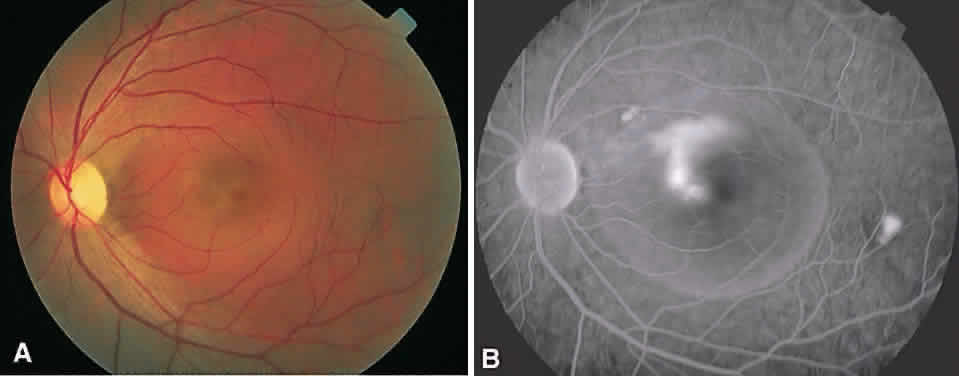
In the DRS, certain high-risk characteristics (HRCs) were identified as being the most accurate prognostic indicators of visual loss. HRCs include neovascularization on or within one disc diameter of the disc (NVD) that is equal to or greater than one fourth to one third of the disc area in extent or any NVD or retinal neovascularization else-where (NVE) associated with preretinal or vitreoushemorrhage (Fig. 1B). For eyes with these clinicalcharacteristics, prompt PRP is recommended. Forpatients with severe or severe nonproliferativediabetic retinopathy (NPDR), as well as for patientsin whom adequate follow-up cannot be ensured, early scatter therapy may be considered.26 The presence of rubeosis iridis or neovascular glaucoma in the setting of PDR, even without HRCs, may warrant PRP.27–29
The precise mechanism of formation of neovascular tissue remains uncertain, as does the mechanism by which PRP induces regression of this tissue. Traditionally, new vessel formation was thought to be a response to a possible angiogenic factor that may be produced by zones of ischemic retina.30 Destruction of these zones by PRP might then eliminate the source of this angiogenic stimulus. Although this approach may not accurately reflect the true pathophysiology involved, it does serve as a helpful model for understanding the proper methods of treatment for this condition. These concepts apply to other proliferative retinopathies as well including central retinal vein occlusion, branch retinal vein occlusion, SC sickle disease, sickle thalassemia, retinopathy of prematurity, Eales' disease, dominant familial exudative retinopathy, and X-linked dominant incontinentia pigmenti. Of note, pregnancy can exacerbate and accelerate all aspects of diabetic retinopathy. It is unusual for pregnant patients without retinopathy at the onset of pregnancy to pro-gress to PDR during their pregnancy. However, patients with preexisting PDR are at greatest risk of visual loss and need to be observed closely and treated aggressively.
Methods
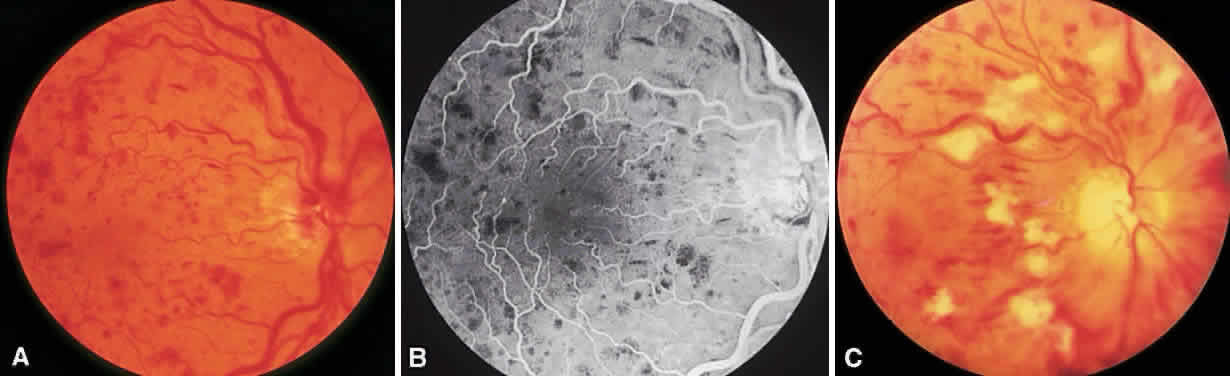
To complete scatter treatment, burns should be applied to the retina beginning at points on an oval defined as two disc diameters above, below, and temporal to the center of the macula, and one disc diameter nasal to the disc, and should extend peripherally at least to the equator (Fig. 2). This approach may help avoid inadvertent macular burns. A report by Blankenship suggests that sparing of the posterior aspect of the fundus may be possible without diminishing the beneficial effects of treatment.31 With the Goldmann lens, a 500-μm spot size is used, whereas with the panfunduscopic lens, a 200-μm spot is used. When vitreous hemorrhage is present, it may be necessary to reduce the size of the spot to 200 μm or to use a krypton or diode laser rather than an argon laser. The Krypton Argon Regression of Neovascularization Study found that krypton and argon were equally effective in inducing regression of NVD.32
Care should be taken to avoid hitting any visible retinal vessels. The power setting should be such that a moderate white retinal coagulation is apparent. The power setting required to achieve this endpoint is recorded for future reference and is defined as the baseline power setting for the treatment session. It frequently needs to be higher for a panfunduscopic lens than for a three-mirror lens.
In general, application should be scattered uniformly, with the distance between burns being one burn diameter. It may be wise to treat inferiorly during the first session because vitreous hemorrhage, should it occur, tends to settle inferiorly. To minimize impairment of the temporal visual field, burns within four to five disc diameters of the disc on the nasal side should be arranged in rows parallel to the nerve fibers. Exposure time should be set at 0.1 or 0.05 second. In general, at least 1600 to 1800 burns should be applied. We usually treat over multiple sessions.
Occasionally PRP fails to induce regression of neovascularization and vision decreases. This can be a result of increased macular edema, focal bleeding from persistent neovascular fronds, retinal detachment, or neovascular glaucoma. Regression of HRCs occurs in 70% of cases within 3 weeks of treatment.33 Not surprisingly, patients who demonstrate a favorable early objective response to laser therapy have a significantly better visual prognosis than those who do not.34 In the case of increasing NVD or persistent NVD with bleeding, supplemen-tal treatment may be helpful. Peripheral retinal cryo-ablation may also be effective in causing regression of NVD or NVE. Cryotherapy is especially helpful in the setting of significant media opacities. About six applications are necessary in each quadrant. If vitreous hemorrhage has occurred and appears to be nonclearing, vitrectomy surgery may be indicated.
DIABETIC MACULAR EDEMA
Diabetic macular edema (DME) is a clinical diagnosis made with the slit-lamp biomicroscope. Intraretinal thickening is divided into focal and diffuse forms. Focal retinal edema is usually caused by specific leaking microaneurysms visible as pinpoint areas of leakage on fluorescein angiography (FA). The diffuse form of macular edema, conversely, usually represents a more widespread disruption of the inner blood-retina barrier. FA typically depicts large areas of leakage. It is important to remember that leakage of dye on FA does not always mean intraretinal thickening is present. Diagnosis of macular edema is based on the clinical examination of the retina.
The Early Treatment of Diabetic Retinopathy Study (ETDRS) was a multicenter, randomized, prospective clinical trial designed to address three main questions: Is laser photocoagulation effective in the treatment of DME? When in the course of diabetic retinopathy is it most effective to initiate PRP? Is aspirin effective in altering the course of diabetic retinopathy? The ETDRS demonstrated that laser photocoagulation for macular edema decreased the risk of moderate visual loss (defined as a doubling of the visual angle) by more than 50% especially in eyes with patterns of macular edema now known as clinically significant macular edema (CSME).35 Focal treatment also increased the chance of moderate visual gain.
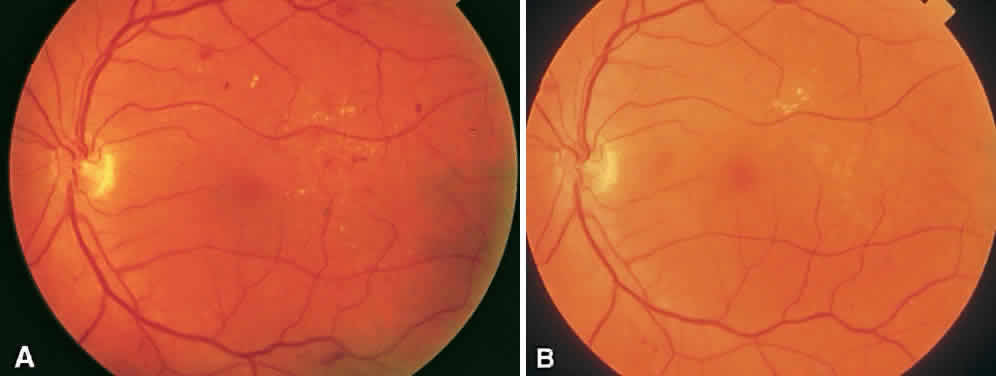
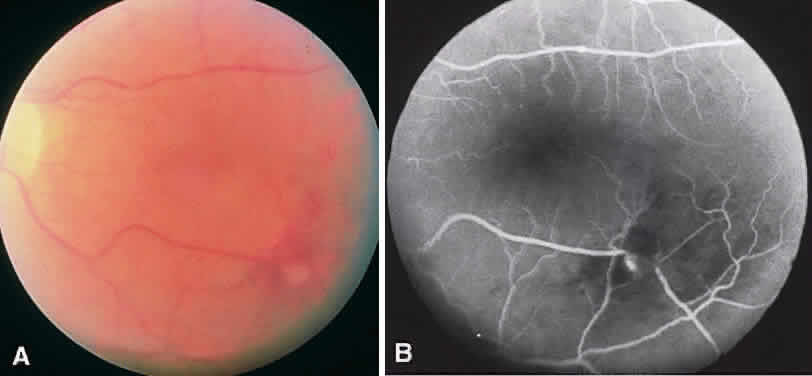
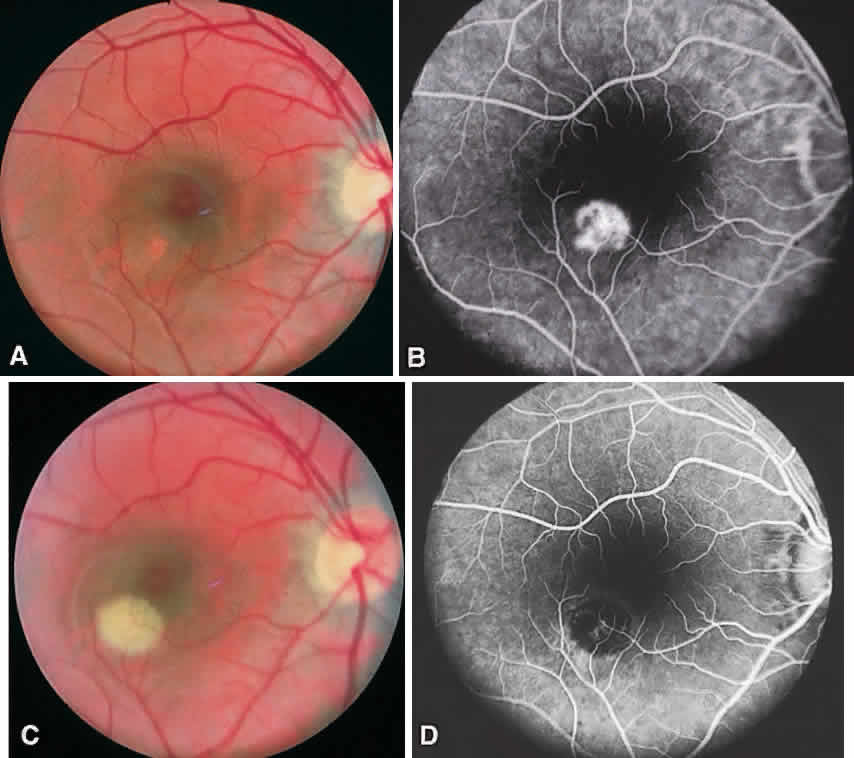
CSME was defined as retinal thickening within 500 μm of the center of the macula, intraretinal hard exudate within 500 μm of the center of the macula associated with adjacent retinal thickening, or retinal thickening greater than one disc area any part of which is within one disc diameter of the center of the macula (Fig. 3A). The beneficial effects of treatment demonstrated in this trial suggest that all eyes with CSME should be considered for focal photocoagulation (see Fig. 3B). Visual acuity was not a factor in determining the presence or absence of CSME and some eyes in the treated group had20/20 visual acuity. Many retinal specialists, how-ever, defer treatment in asymptomatic patients with20/20 visual acuity except when hard exudate is encroaching on the fovea.
When CSME is present, fluorescein angiography is helpful in ruling out macular ischemia as a cause for decreased vision. If significant perifoveal capillary nonperfusion is present, then macular photocoagulation is associated with a higher risk of producing an immediate and permanent reduction in visual acuity. If photocoagulation is performed in such cases, care should be taken to avoid directly treating the few remaining perifoveal capillaries.
Methods
Various treatment strategies have been devised for photocoagulation of DME. Although the optimal method remains uncertain, most adhere to the basic principles described in the ETDRS. The argon green laser is widely used, although evidence now suggests that other wavelengths, such as dye yellow and orange, krypton red, and the diode, are comparable.36–39 A pretreatment fluorescein angiogram is generally used during photocoagulation to identify treatable lesions. Focal laser treatment involves direct laser treatment to all leaking microaneurysms.
When directly treating a microaneurysm, a 50- or 100-μm burn with 0.1-second duration is used, and one usually attempts to induce a slight change in the color of the lesion. Use of the 50-μm spot size may be associated with a higher risk of rupturing Bruch's membrane and resultant choroidal neovascularization (CNV), so very low energy levels should be used initially.40 Initial treatment is directed at lesions located within two disc diameters of the center of the macula, but not closer than500 μm from its center. However, if vision is lessthan 20/40 and follow-up examination reveals persistent CSME, additional treatment of lesions up to 300 μm from the center is recommended.
Areas of diffuse leakage or nonperfusion within two disc diameters of the center of the macula are treated with a grid pattern. Diode laser has been shown to be as effective as argon laser in the treatment of diffuse DME.41 The goal of treatment in such cases is to produce a burn of light to moderate intensity. A 100- to 200-μm spot size is used, and burns are typically spaced one burn-width apart. Burns may be placed in the papillomacular bundle but, again, not closer than 500 μm from the center of the macula at the first treatment. For patients with a combination of focal and diffuse edema, a modified grid technique may be employed.42
In addition to the development of CNV mentioned earlier, other complications can occur after focal macular photocoagulation. Permanent visual loss may ensue after treatment. Causes includehemorrhage, perifoveal capillary occlusion, enlargement of photocoagulation scars to involve the fovea, marked lipid exudation as edema resolves, or in-advertent foveal burns. Paracentral scotomataare a frequent side effect, especially after heavy treatment.
Some patients present with CSME in the presence of PDR with HRC. Because PRP can exacerbate preexisting macular edema, it is generally advisable to initiate focal or grid treatment prior to or along with PRP. Performing nasal PRP first and completing the PRP is additional sessions may help prevent a worsening of the macular edema.
Regarding the use of aspirin, the ETDRS found that aspirin use did not affect the progression of retinopathy. Aspirin did not increase the risk for vitreous hemorrhage, the duration of or severity of vitreous hemorrhage, nor the rate of pars plana vitrectomy. Visual outcomes were not affected by aspirin use.43 Therefore, there is no ocular contradiction to the use of aspirin in patients with diabetic retinopathy.