1. Kijlstra A: The value of laboratory testing in uveitis. Eye 4:732, 1990 2. Rothova A, Buitenhuis HJ, Meenken C et al: Uveitis and systemic disease. Br J Ophthalmol 76:137, 1992 3. Asdourian GK, Goldberg MF, Busse BJ: Peripheral retinal neovascularization in sarcoidosis. Arch Ophthalmol 93:787, 1975 4. Graham EM, Stanford MR, Shilling JS et al: Neovascularization associated with posterior uveitis. Br J Ophthalmol 71:826, 1987 5. Engel HM, Green WR, Michels RG et al: Diagnostic vitrectomy. Retina 1:121, 1981 6. Barondes MJ, Sponsel WE, Stevens TS, Plotnik RD: Tuberculous choroiditis diagnosed by chorioretinal endobiopsy. Am J Ophthalmol 112:460, 1991 7. Freeman WR, Wiley CA, Gross JG et al: Endoretinal biopsy. Indications, utility and techniques. Ophthalmology 96:159, 1989 8. Ohno S, Char DH, Kimura SJ, O'Connor GR: Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol 83:735, 1977 9. Gass JDM, Sever RJ, Grizzard WS et al: Multifocal pigment epithelial detachments by reticulum cell sarcoma: A
pathognomonic funduscopic picture. Retina 4:135, 1984 10. Michelson JB, Michelson PE, Bordin GM et al: Ocular reticulum cell sarcoma: Presentation as retinal detachment with
demonstration of monoclonal immunoglobulin late chains on the vitreous
cell. Arch Ophthalmol 99:1409, 1981 11. Innis MA, Gelford DH, Sninsky JJ, White TA: PCA Protocols. New York, Academic
Press, 1990 12. Wiedbrauk DL, Werner JC, Devon AM: Inhibition of the polymerase chain reaction by aqueous and vitreous fluids (abstr). Invest Ophthalmol Vis Sci 2012:1688, 1994 13. Foster CS: Cataract surgery and intraocular lens implantation in patients
with intermediate uveitis. In Böke WRF, Manthey KF, Nussenblatt
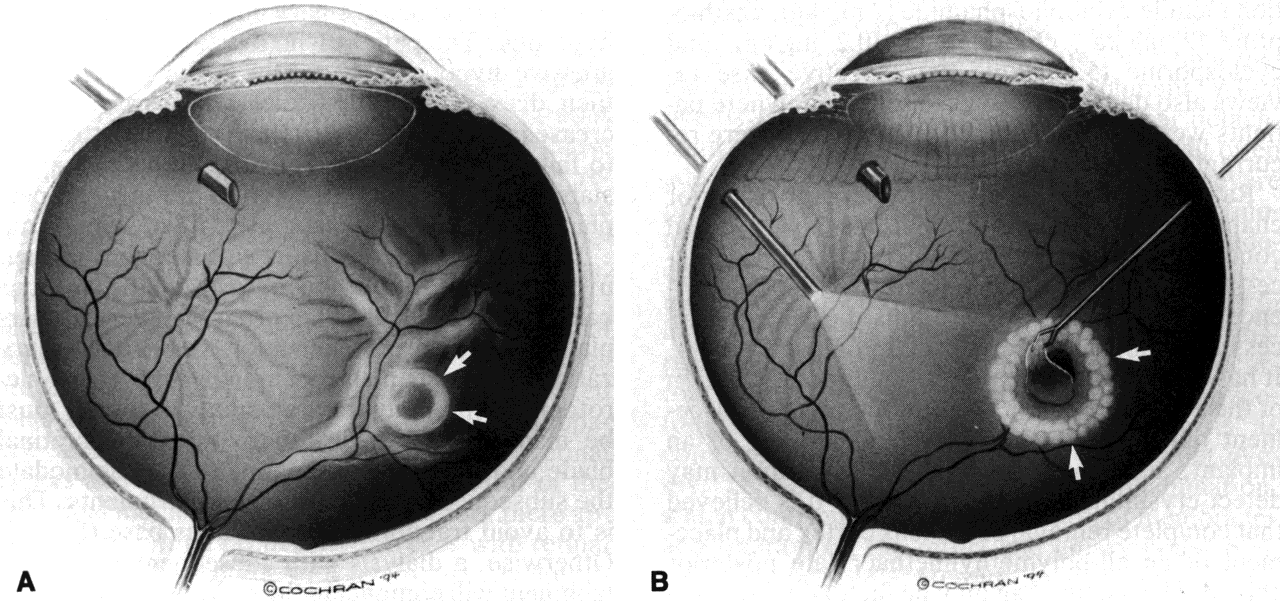
RB (eds): Intermediate Uveitis, pp 212–218. Basel, Karger, 1992 14. Foster CS, Fong LP, Singh G: Cataract surgery and intraocular lens implantation in patients with uveitis. Ophthalmology 96:281, 1989 15. Tessler HH, Farber MD: Intraocular lens implantation versus no intraocular lens implantation in
patients with chronic iridocyclitis and pars planitis: A randomized
prospective study. Ophthalmology 100:1206, 1993 16. Kaufman AH, Foster CS: Cataract extraction in patients with pars planitis. Ophthalmology 100:1210, 1993 17. Dangel ME, Stark WJ, Michels RG: Surgical management of cataract associated with chronic uveitis. Ophthalmic Surg 14:145, 1983 18. Ridley H: Cataract surgery in chronic uveitis. Trans Ophthalmol Soc UK 85:519, 1965 19. Foster CS, Fong LP, Singh G: Cataract surgery and intraocular lens implantation in patients with uveitis. Ophthalmology 96:281, 1989 20. Apple DJ, Mamalis N, Steinmetz RL et al: Phacoanaphylactic endophthalmitis associated with extracapsular cataract
extraction and posterior chamber intraocular lens. Arch Ophthalmol 102:1528, 1984 21. Smith RE, Kokoris N, Nobe JR et al: Lensectomy-vitrectomy in chronic uveitis. Trans Am Ophthalmol Soc 81:261, 1983 22. Flynn HW, Davis JL, Culbertson WW: Pars plana lensectomy and vitrectomy for complicated cataracts in juvenile
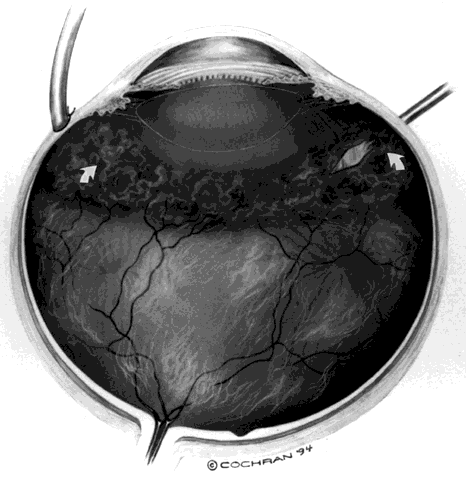
rheumatoid arthritis. Ophthalmology 95:1114, 1988 23. Diamond JF, Kaplan HJ: Uveitis: Effect of vitrectomy combined with lensectomy. Ophthalmology 86:1320, 1979 24. Diamond JG, Kaplan HJ: Lensectomy and vitrectomy for complicated cataract secondary to uveitis. Arch Ophthalmol 96:1798, 1978 25. Werry H, Honegger H: Pars plana vitrektomic bei chronischer uveitis. Klin Monatsbl Augenheilkd 191:9, 1987 26. Belfort R, Nussenblatt RB, Lottemberg C et al: Spontaneous lens subluxation in uveitis. Am J Ophthalmol 110:714, 1990 27. Mieler WF, Will BR, Lewis H, Aaberg TM: Vitrectomy in the management of peripheral uveitis. Ophthalmology 95:859, 1988 28. Mieler WF: Closed vitrectomy and chronic uveitis. Semin Ophthalmol 4(1):1, 1989 29. Michelson JB, Nozik RA: Surgical Treatment of Ocular Inflammatory Disease. Philadelphia, JB
Lippincott, 1988 30. Aaberg TM, Cesarz TJ, Flickinger RR Jr: Treatment of pars planitis. I. Cryotherapy. Surv Ophthalmol 22:120, 1977 31. Aaberg TM, Cesarz TJ, Flickinger RR: Treatment of peripheral uveoretinitis by cryotherapy. Am J Ophthalmol 75:685, 1973 |