| General anesthesia is a state of unconsciousness that includes analgesia
and amnesia with or without muscle relaxation. The process of anesthesia
may begin with premedication. Vital signs are monitored from the
time of administration of depressant medication until recovery of consciousness. The
anesthesiologist evaluates the patient postoperatively
to ensure no complications have occurred. PREMEDICATION Premedication is given to decrease the child's anxiety, provide sedation, and
facilitate both separation from parents and acceptance of
mask or intravenous induction of anesthesia. Premedication should not
be given to every child. Children younger than 6 months of age should
not routinely receive premedication, nor should any child with upper airway
obstruction (e.g., large tonsils, a history of long respiratory
pauses while asleep), because sedation may easily depress spontaneous
ventilation in these patients. In addition, patients with intracranial
pathology (increased intracranial pressure) or end-stage liver or renal
disease may experience an excessive degree of sedation. However, the
anxious 1- to 5-year-old child will most likely benefit from sedation
before coming to the operating room. Midazolam administered intranasally (0.2 to0.3 mg/kg) or orally as elixer (0.3 to 0.5 mg/kg
with maximum of 20 mg) has largely replaced oral
diazepam (0.1 to 0.2 mg/kg) as the preferred agent for sedation in outpatient
pediatric surgery. The onset of maximum effect of intranasal midazolam
is rapid (10 to 15 minutes), and postoperative sedation is minimal. However, there
is significant potentiation of the respiratory depressant
effects of narcotics by midazolam. Fentanyl (1 to 2 μg/kg) may
be given intravenously immediately before induction because of the
rapid onset of its sedative and analgesic effects if an IV is present. Preoperatively, antibiotics may be administered as prophylaxis against
infection, especially for SBE in a child with congenital heart disease. Antibiotics
may be administered intravenously immediately after induction
of anesthesia and placement of the intravenous catheter and before
intubation and start of surgery. Ampicillin, 50 mg/kg, is recommended; if
an allergy to penicillin or cephalosporin antibi-otics exists, clindamycin (20 mg/kg) or
vancomycin(20 mg/kg) is prescribed. Vancomycin
can cause hypotension, so it must be administered over a 1-hour period (see Table 2). REGIONAL ANESTHESIA Some children may undergo ocular surgery with regional nerve blocks and
sedation, but most young patients require a general anesthetic. If regional
nerve blocks and supplementary sedation are administered, an anesthetist
should always monitor the child throughout the procedure because
sedation may produce complete loss of consciousness and airway reflexes. The
same preoperative evaluation and fasting requirements, use
of monitoring equipment, and written documentation are required for a
procedure that is performed with intravenous sedation instead of general
anesthesia.8 EQUIPMENT Noninvasive continuous intraoperative monitoring includes auscultation
with a precordial stethoscope, pulse oximetry, and an electrocardiogram, as
well as noninvasive blood pressure and temperature determinations. End-tidal
carbon dioxide and concentrations of potent inhalation agents
are also monitored continuously when an endotracheal tube or LMA
is in place. A peripheral nerve stimulator should be used to monitor neuromuscular
function whenever neuromuscular blocking drugs are administered. Temperature (skin, rectal, or esophageal) is routinely monitored. Some
heat is lost through evaporation from the lungs and radiation from the
large body surface of the infant. Measures to decrease heat loss include
increasing the temperature of the operating room, using radiant heat
lamps, warming the inspiratory gases with a heated humidifier, warming
intravenous fluids, and keeping the child covered. Hypothermia (temperature
below 35°C) can increase the potency of several drugs administered
during anesthesia (i.e., muscle relaxants, narcotics, barbiturates, and
inhaled anesthetics) and depress respiratory drive, especially
in infants. Iatrogenic hyperthermia also occurs in small children
covered with plastic drapes for a long period of time (more than 30 minutes) and
should be avoided. Use of a cooling blanket and other active
measures should be instituted if the child's temperature reaches 38°C. An intravenous catheter is usually placed after induction of anesthesia
when the child is asleep. The intravenous catheter should be carefully
secured so it will be available for use after surgery. Stabilization
of the child's arm or foot to an arm board is advisable. Principles
guiding the administration of intravenous fluid are discussed later
in this chapter. The type of airway equipment used depends on the age and size of the child. The
endotracheal tube chosen for most ocular surgery is a curved
tube called a RAE tube, which is named for its developers Ring, Adair, and
Elwyn. The advantage of this endotracheal tube is that it will not
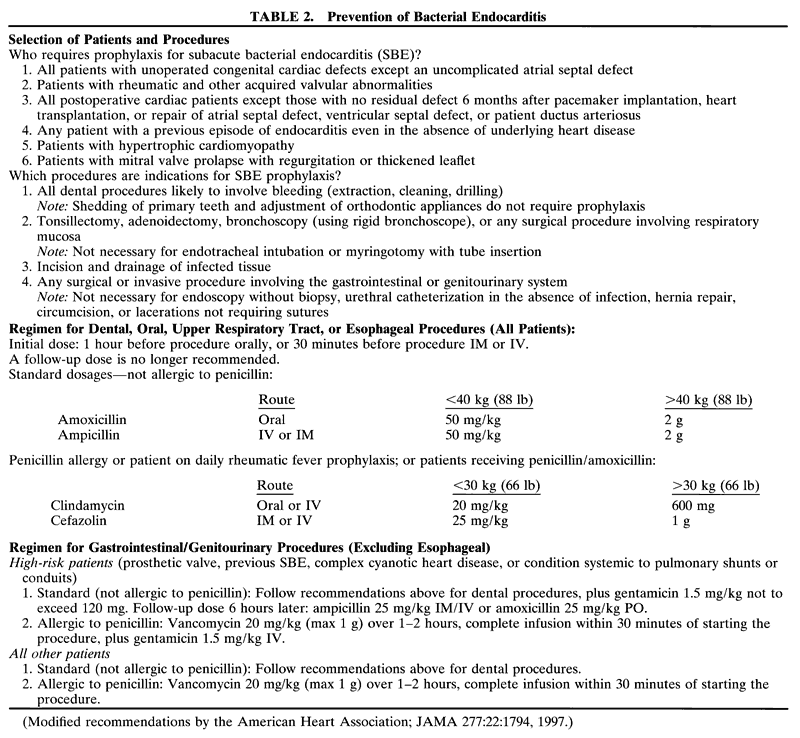
disturb the surgical field (Fig. 1). These tubes are a fixed length from the bend at the lip and thus accidental
extubation or right main-stem intubation may occur after extending
or flexing the patient's head. 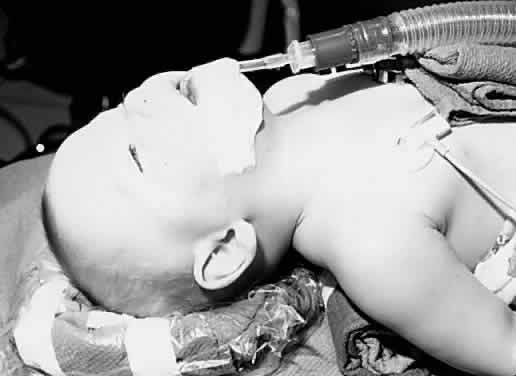 Fig. 1. An infant is positioned for ophthalmic surgery. A preformed curved endotracheal
tube is in place. Fig. 1. An infant is positioned for ophthalmic surgery. A preformed curved endotracheal
tube is in place.
|
An LMA is commonly used for eye surgery (Figs. 2 and 3). This mask is placed in the pharynx above the epiglottis, so it does
not protect the lungs from aspiration. The LMA was developed by A. Brain
in England and became popular in the United States in the 1990s. A
reinforced form of LMA is also available. 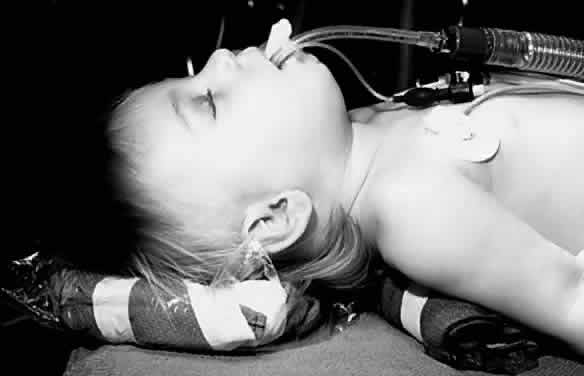 Fig. 2. A laryngeal mask airway with flexible reinforced tube is in place in this
infant. Fig. 2. A laryngeal mask airway with flexible reinforced tube is in place in this
infant.
|
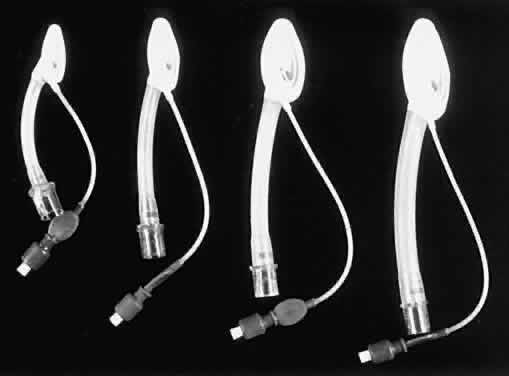 Fig. 3. Examples of laryngeal mask airways. Fig. 3. Examples of laryngeal mask airways.
|
INDUCTION Induction of anesthesia may be through a mask with a potent inhalational
agent (halothane, sevoflurane). A pleasant flavor may be applied to
the mask with Chapstick to make the mask more acceptable to the child. Other
induction methods include intravenous administration of 2.5% sodium
thiopental (4 to 8 mg/kg), propofol (2 to 3 mg/kg), or rectal administration
of methohexital sodium (20 to30 mg/kg). Intramuscular injection
of ketamine (1 to 8 mg/kg) may facilitate induction but nystagmus
is common and may make ocular surgery difficult. Light levels of anesthesia
may produce poor operating conditions. Bradycardia with or without
hypotension may occur during inhalation induction with halothane
and is caused by the cardiac effect of halothane. This is less common
with sevoflurane than halothane. Bradycardia is treated by administering
atropine (10 to 20 μg/kg), intravenous fluids, intravenous calcium, or
epinephrine, and discontinuing the anesthetic agent. Because intravenous
medication can facilitate securing the airway and establishing
cardiovascular stability, an intravenous catheter should be placed
as soon as possible after the child is asleep. In children, veins in the
lower extremities or scalp may be used in addition to those in the
upper extremities. Intramuscular injection of ketamine is usually reserved for uncooperative
children who have not been sedated adequately by premedication withmidazolam
or for older children (15 to 20 years) who are unable to control
themselves in the operating room setting (e.g., the severely mentally
handicapped). Low-dose ketamine (1 to 3 mg/kg IM) may quiet the child
sufficiently to accept intravenous or mask induction. Complications
of intramuscular ketamine include a possible increase in intraocular
pressure (IOP), increased secretions, loss of airway reflexes, airway
obstruction, and postoperative hallucinations. Anticholinergic should
be administered with ketamine to decrease secretions. INTUBATION Some surgical procedures can be performed without an endotracheal tube
in place, such as examination under anesthesia or probe and irrigation
of the nasolacrimal duct, especially if the procedure is brief (less
than 5 minutes; Figs. 4 and 5). However, intubation with a properly sized endotracheal tube is a routine
part of most procedures involving anesthetics, especially in younger
patients undergoing surgery on the face, and this can be safely performed
even for brief procedures. Intubation should be performed when
the child is deeply anesthetized and has little muscular tone. Neuromuscular
blocking drugs are optional. Common complications ofmask anesthesia
include airway obstruction, laryngospasm, silent aspiration, and
hypotension from deep anesthesia. Protecting the airway with a properly
sized endotracheal tube can prevent most of these problems. 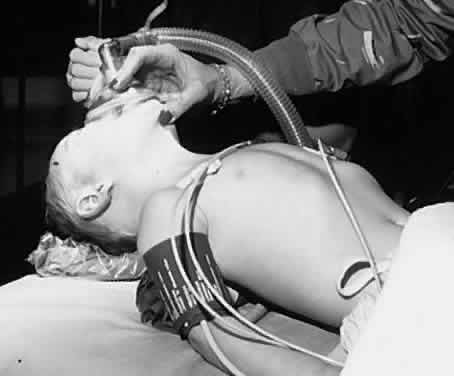 Fig. 4. A child is positioned for a brief procedure with a mask anesthetic. Fig. 4. A child is positioned for a brief procedure with a mask anesthetic.
|
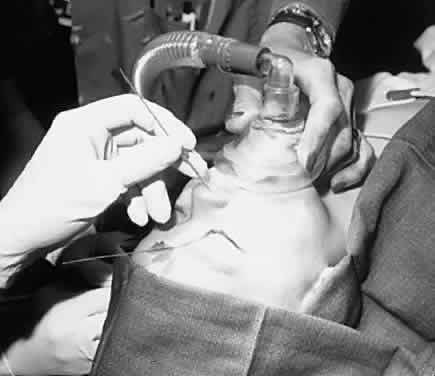 Fig. 5. Mask is positioned in this manner for a probe and irrigation of the nasolacrimal
duct. Fig. 5. Mask is positioned in this manner for a probe and irrigation of the nasolacrimal
duct.
|
An LMA may be used instead of an endotracheal tube (see Figs. 2 and 3). This type of airway is less stimulating than an endotracheal tube and
is easier to place; however, aspiration of gastric fluid into the lungs
may still occur. Insertion of an LMA in an anesthetized child does
not increase IOP as tracheal intubation does. MAINTENANCE ANESTHETICS Maintenance anesthetic drugs include intravenous propofol (50 to 300 μg/kg/min), remifentanil (0.1 to 0.4 μg/kg/min), or potent inhalational
agents (e.g., halothane, isoflurane, and sevoflurane) with analgesic, muscle
relaxant, and antiemetic added when needed. N2O is often added but there is some evidence that this may contribute to
postoperative nausea and vomiting. Fentanyl (1 to 2 μg/kg) may be
administered to those children who are likely toexperience postoperative
pain. Other analgesics such as morphine (0.05 to 0.1 mg/kg), acetaminophen (10 to 30 mg/kg), or ketorolac (0.8 mg/kg with maximum of 30 mg) may
also be used. Nondepolarizing neuromuscular relaxants may be administered
as well. Antiemetic sedatives such as droperidol (10 to 75 μg/kg) may
be administered. Other commonly used antiemetics include
ondansetron (0.1 mg/kg with maximum of 4 mg) and metoclopramide (0.15 to 0.2 mg/kg) IV. Because
the choice of drug may differ depending on
the duration of surgery, careful estimation of surgical time helps the
anesthesiologist to make an appropriate choice, thus increasing the chance
of a prompt, smooth emergence from anesthesia when the surgery has
been completed. SUCCINYLCHOLINE Succinylcholine is a depolarizing neuromuscular blocking drug that has
a rapid onset (less than 1 minute) and a short duration (less than 5 minutes) of
effect when 1 to 2 mg/kg is administered intravenously to a
patient with normal plasma cholinesterase function. This drug is used
to facilitate intubation for emergency procedures in patients with a
full stomach or to treat laryngospasm; it has many undesirable side effects: arrhythmias, bradycardia, sinus arrest or asystole, hyperkalemia, noticeably
increased muscle tone in the masseter, potential trigger
of malignant hyperthermia, myoglobinemia, myoglobinuria, increase in
intragastric pressure, and transient increase of IOP. The mechanism for
the increase in IOP is unclear; possible causes include a direct effect
on choroidal blood volume, increased formation of aqueous humor, or
tonic response of the extraocular muscles. Muscle fasciculation is not
the cause. The increase in IOP may be as great as 10 to 20 mmHg and
lasts for 4 to 6 minutes. The results of forced duction tests may be
altered for 20 to 30 minutes after a patient received succinylcholine. Vitreous
humor may be extruded from an open globe if IOP increases after
succinylcholine. Enophthalmos may occur after succinylcholine administration; this
too resolves with time, but it may make an intraocular
procedure more difficult to perform. EMERGENCE Emergence from anesthesia should be smooth. Coughing on the endotracheal
tube can cause an increase in both venous pressure and IOP and can be
avoided by extubating the child while he or she is still deeply anesthetized
before all airway reflexes have returned. Potential complications
of extubation are airway obstruction including laryngospasm, hypoxemia (even
without airway obstruction), and aspiration of secretions
or gastric contents. Infants younger than 6 months of age should not be
extubated while anesthetized. They are more likely to have airway obstruction
and hypoxemia than older infants and children are because of
their relatively large tongues and irregular breathing patterns or breath
holding. Some anesthesiologists advocate intravenous lidocaine (1 to 1.5 mg/kg) at
the com-pletion of the procedure to reduce incidence
of coughing on the endotracheal tube. This drug increases the depth of
anesthesia and may be effective at blocking airway reflexes. Laryngospasm
can precipitate postobstructive pulmonary edema that may require
oxygen, continuous positive airway pressure (CPAP), and treatment with
diuretics (furosemide [Lasix] 0.1 to 0.2 mg/kg). FLUID MANAGEMENT Fluids to be administered perioperatively are divided into four categories: maintenance, deficit, third-space loss, and blood replacement. The
last two categories are unnecessary for most elective ocular procedures. Hourly
maintenance fluid is calculated by the child's weight: 4 ml/kg
for the first 10 kg, plus 2 ml/kg for each kilogram between 10 and 20 kg, plus 1 ml/kg
for each kilogram over20 kg (Table 3). The ideal maintenance fluid to provide for metabolic needs is 2.5% to 5% dextrose
in 0.25 normal saline (NS); however, usually Ringer's
lactate (RL) is administered intraoperatively to provide both maintenance
as well as deficit fluid replacement to ensure that adequate sodium
is provided. Glucose solutions may be administered to infants less
than 2 months of age intraoperatively because hypoglycemia is otherwise
undetected under general anesthesia. In adults and healthy children
who have fasted for only 3 hours, hypoglycemia is unlikely to occur
during an anesthetic of short duration.9 Endogenous catecholamine release during induction of anesthesia and surgery
usually increases blood glucose intraoperatively. Administration
of excessive amounts of dextrose is also undesirable, because osmotic
diuresis may result.
 All patients have obligate fluid loses due to respiration, urination, and
sweating. This loss is a large percentage of extracellular fluid volume
in the child. Therefore, it is necessary to replace the fluid deficit
that has occurred while fasting. Deficit fluid is calculated by multiplying
the maintenance volume by the number of hours of fasting (i.e., a 10-kg
child fasting for 8 hours requires: [10 kg × 4 ml/kg/hr] × 8 hr = 320 ml). Half of this deficit should be
replaced in the first hour of surgery, and the remainder during the second
and third hours. RL is usually administered for this purpose. Because
nausea and vomiting are common postoperative complications after
extraocular muscle surgery, postoperative dehydration may be avoided
or decreased by administering all the fluid deficit in the perioperative
period. Acute sequestration of fluid to a nonfunctional interstitial compartment, also
known as third-space loss, is usually minimal for ocular surgery
but greater for thoracic or abdominal procedures. The estimated loss (1 to 10 ml/kg) is
added to the maintenance rate using RL. Blood loss must be replaced with clear fluid (RL or NS) at a ratio of 2:1 or 3:1, or
with albumin, packed red blood cells, or whole blood at
a lower ratio of 1:1 replacement. Which fluid is chosen depends on the
expected total amount of blood loss, measured blood loss, availability
of blood products, and minimum hematocrit allowable for the individual
patient. A fluid challenge (RL or albumin) may be given in 10 ml/kg
increments. |