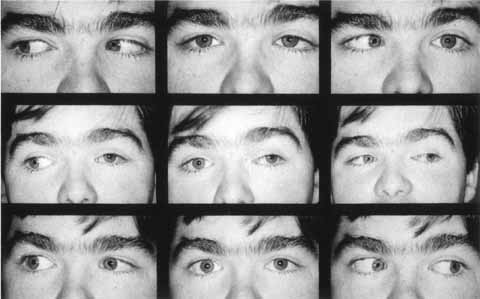
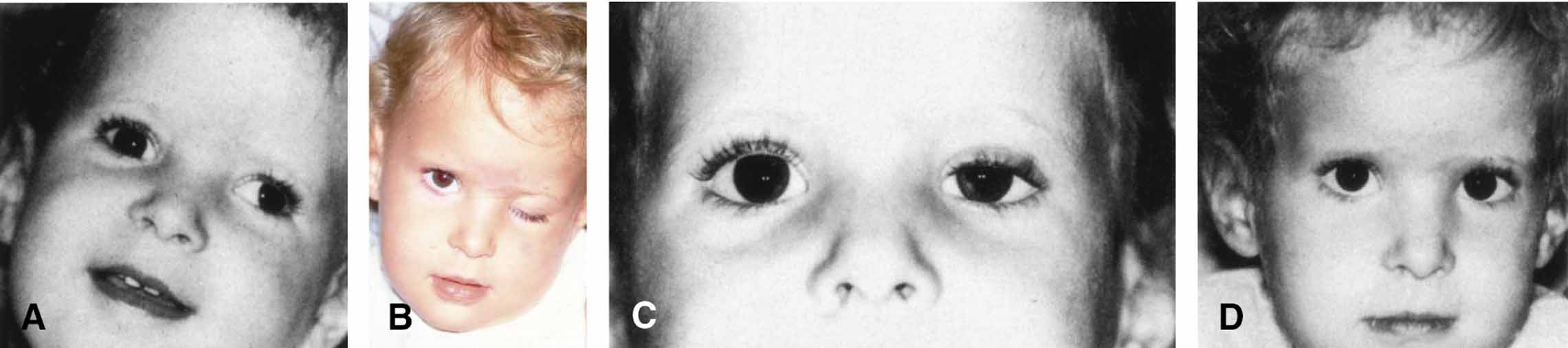
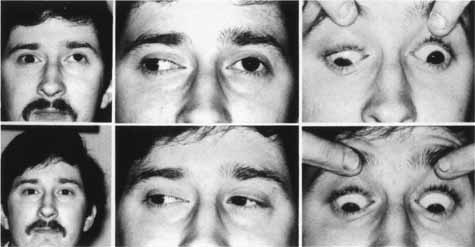
|
LOCATION
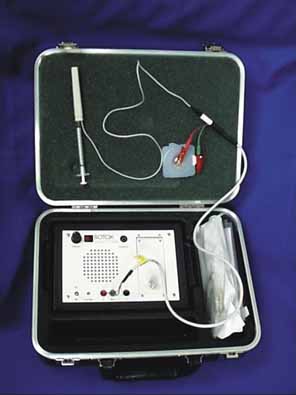
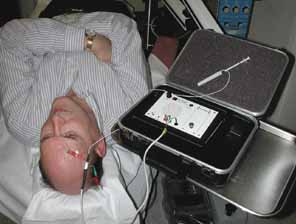
The patient should be made comfortable in the supine position (Fig. 3). Although this can be performed in an office chair, a flat examination table or similar setup is advisable both for patient comfort and stability of the head during the procedure. The room should be as free as possible from electrical signals that can interfere with the audio EMG recording device. Certain fluorescent fixtures and overhead wiring produce signals that can be picked up by the recorder, thereby interfering with assessment during the injection process. Loud background noise often occurs when the EMG device is turned on prior to inserting the needle into the conjunctiva. However, as soon as the needle touches the conjunctiva, this noise is greatly diminished. If this does not occur, then accurate localization of the muscle will be difficult. In this situation, reducing the illumination or turning off nonessential lights can be helpful.
PREINJECTION PREPARATION
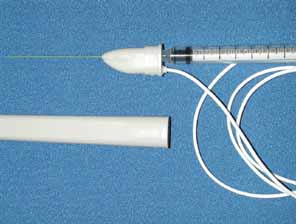
The dosage to be administers should be calculated (Table 2). Nonpreserved saline 0.9% is used to mix the Oculinum so that the desired number of units to be given are in a volume of 0.1 mL or less. Two milliliters of the reconstituted fluid is drawn into a tuberculin syringe; the monopolar electrode needle should not be used for this process. The 27-gauge, 1½-inch, Teflon-coated, monopolar electrode needle is then attached to the end of the tuberculin syringe. The total volume in the tuberculin syringe is reduced to to one-tenth of a milliliter by injecting the excess drug through the needle end. Because of the size of the needle and the small volume used, the needle barrel must be loaded with the drug to avoid undermedicating the patient. The location of the attachment of the wire onto the needle hub can be used as a reference point to determine the position of the needle bevel.
TABLE 2. Botulinum Toxin Dosage for Horizontal and Vertical Rectus Muscles (in
units)
| Deviation Amount | Adults | Children | |||
| in △ | (> 12 yr) | < 6 kg | 6–9 kg | 10–12 kg | > 12 kg |
| < 20 | 1.25–2.5 | 1.25 | 1.25 | 1.25 | |
| 20–50 | 2.5–5 | 1 | 1.25 | 1.25–1.75 | 1.25–2.5 |
△, prism diopter. (Scott AB, Magoon EH, McNeer KW et al: Botulinum treatment of childhood strabismus. Ophthalmology 97:1434, 1990 Published courtesy of Ophthalmology)
A pediatric electrocardiographic electrode is then attached to the patient's forehead, making sure that a good contact is present (see Fig. 3). It should be placed medially for the medial rectus and laterally for the lateral rectus. If not already done, the patient should be informed that an audio signal will be used during the procedure so that he or she will not become startled when the amplifier is turned on. Next, turn on the amplifier and test the connections by touching the needle tip to the conjunctiva. Any background noise should become extinguished and an audible click should be heard. If this does not happen, the ground connection to the needle hub should be checked and possible interference from overhead lights, etc., investigated. This is also the time to make sure that adequate topical anesthesia has been achieved.
INJECTION TECHNIQUE
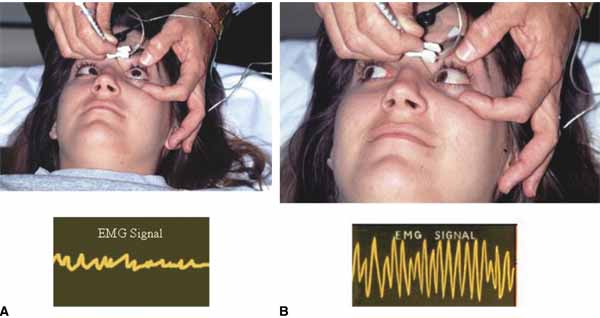
Before beginning, it should be explained to the patient that he or she will have to move the eyes into various gaze positions during the procedure. Therefore, it is important that the patient keep both eyes open and fixed on some target. It is helpful to have an assistant during the injection procedure. Standing at the head of the patient, the lids are held open with the fingers. The patient should look away from the field of action of the injected muscle, for example, when injecting the medial rectus have the patient begin by looking laterally with that eye (Fig. 4). The assistant can use a hand or other fixation target for this purpose. The patient should be reminded to keep both eyes open so that fixation is maintained.
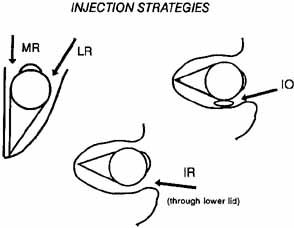
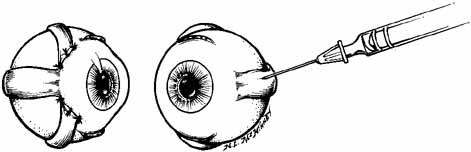
With the bevel facing the muscle, the needle is inserted through the conjunctiva 8 to 10 mm from the limbus. Once through the conjunctiva, the needle is advanced several more millimeters until the tip is beyond the equator. The assistant should move the fixation target slowly to the primary position so that the injection-muscle EMG signal can be activated. If the medial rectus is being injected, continue to advance the needle straight posteriorly aiming for the optic canal (Fig. 5). When injecting the lateral rectus the syringe has to be angled toward the ear at approximately a 45-degree angle to avoid touching the periosteum of the lateral orbital wall with the needle point, which is quite uncomfortable (see Fig. 5). A slight angle is also necessary when injecting the superior rectus. The inferior rectus is often easier to inject through the lower lid rather than the conjunctiva. As the needle is advanced and while listening to the EMG signal, continue to advance until a loud crackling noise occurs. If uncertainty exists as to whether this is from the appropriate muscle, have the patient slowly look out of the field of action of the muscle to be injected. The signal should decrease. Care must be taken to ensure that slow eye movements are made. This may be somewhat difficult because the patient may be anxious or apprehensive and may experience some discomfort. When the electrode is at the appropriate location, the reconstituted toxin is injected slowly. The EMG signal should diminish at this point as the tissue is pushed away from the tip by the entering fluid. Allow the needle to remain in this position for 10 to 15 seconds and then slowly withdraw the needle. The patient should be checked for signs of hemorrhage or other potential complications.
POSTINJECTION MANAGEMENT
Once finished, the patient should be asked to sit up on the edge of the examination table but should not be allowed to stand until postinjection anxiety and bradycardia have subsided. Often the patient is so relieved that the process is over that an episode of lightheadedness or even syncope can occur. One to two minutes is usually all that is necessary at this time. It is not necessary to patch the eye or use antibiotics unless there is some concern about the injection process. The patient should be informed that topical anesthesia has been applied to the eye and ocular manipulation for the next 30 to 45 minutes should be discouraged. The patient should be observed for 5 to 10 minutes to make sure that no retrobulbar bleeding has occurred. Acetaminophen should suffice for any postinjection discomfort.