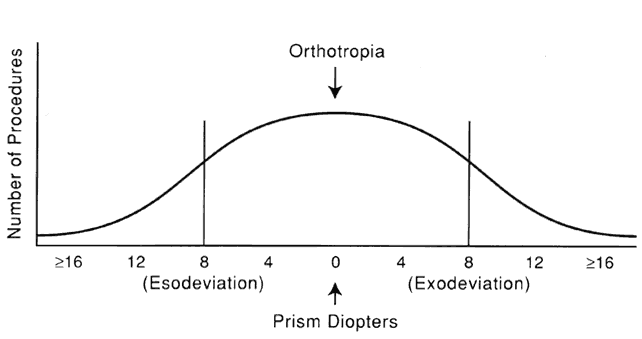
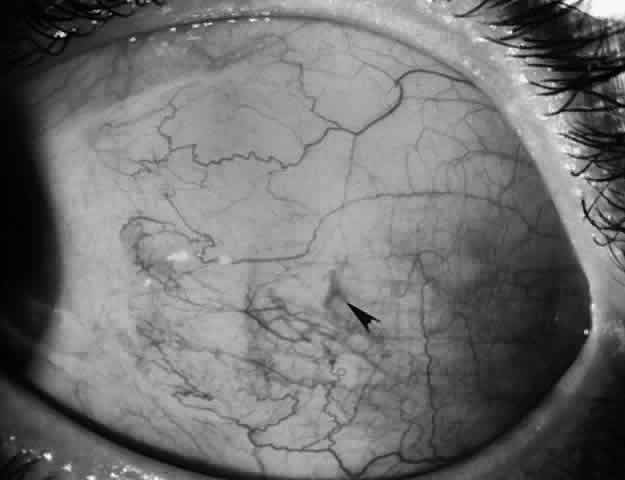
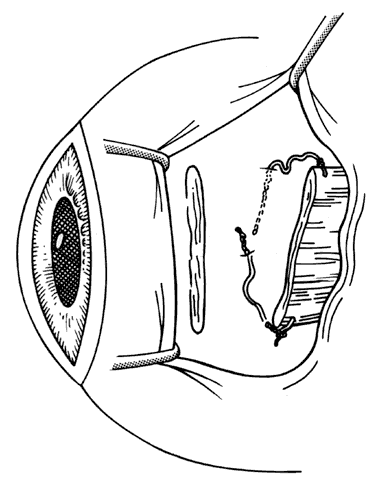
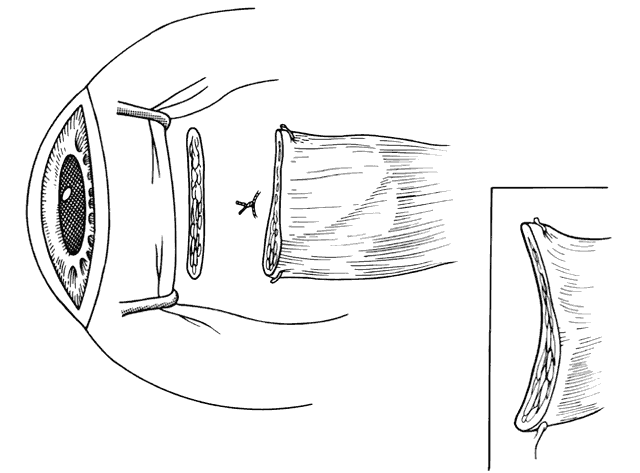
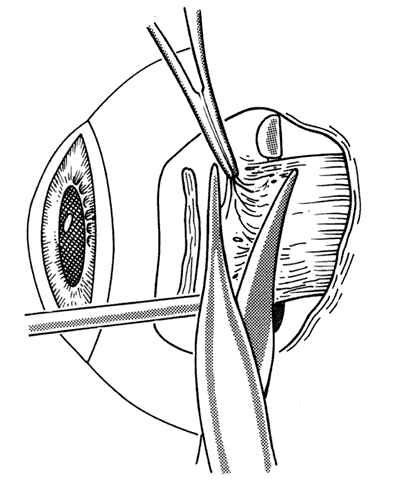
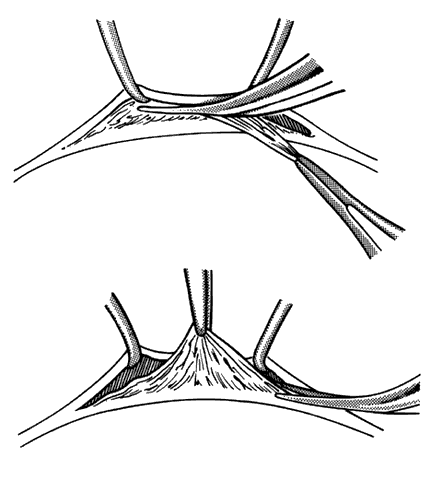
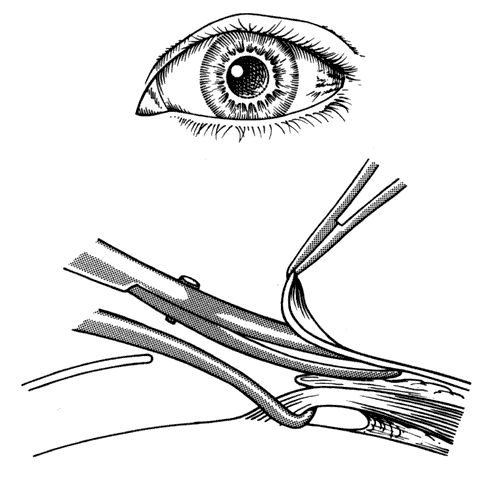
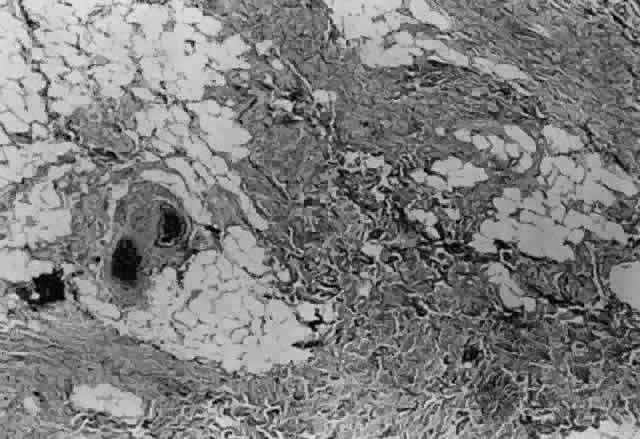
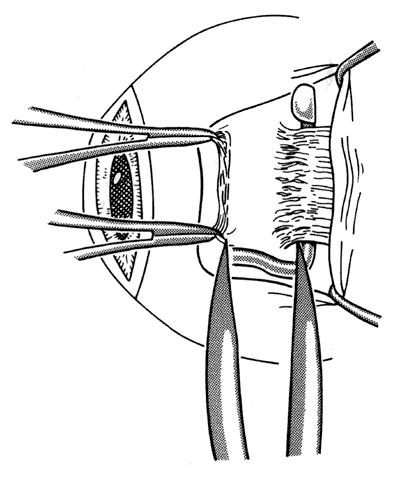
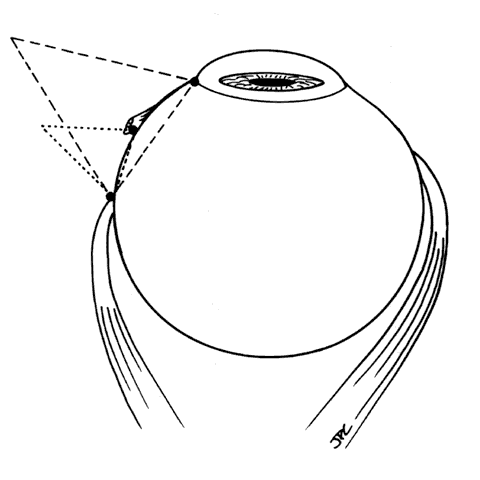
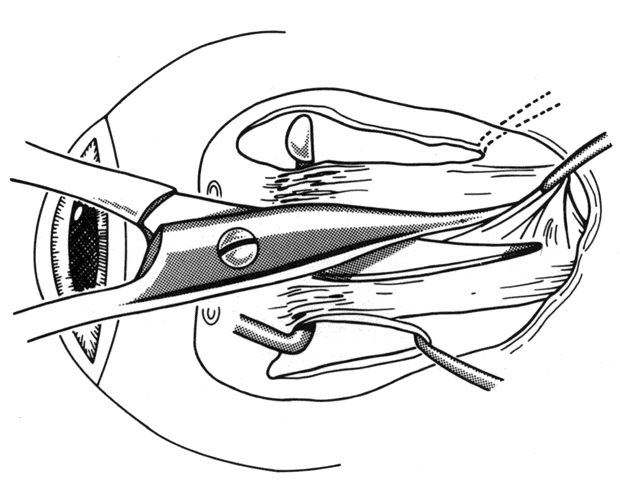
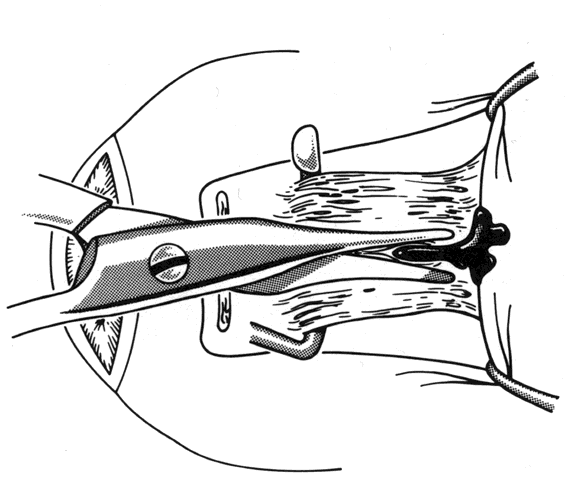
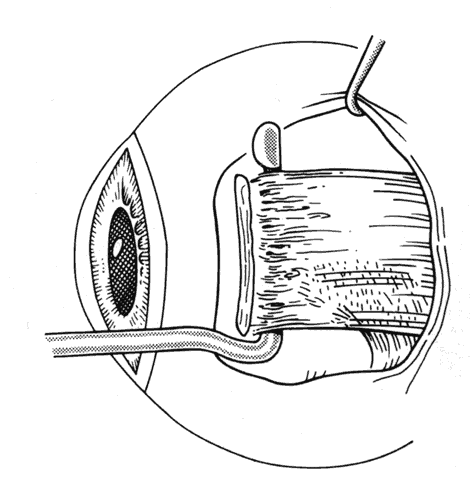
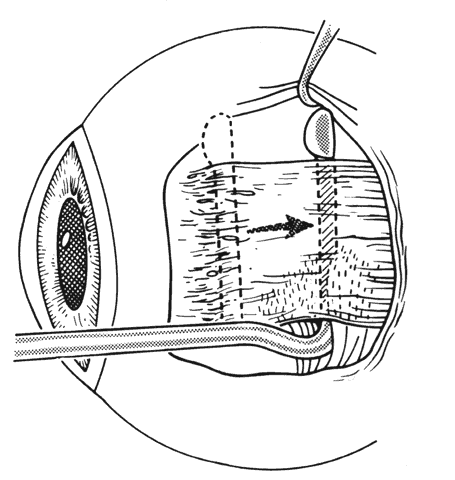
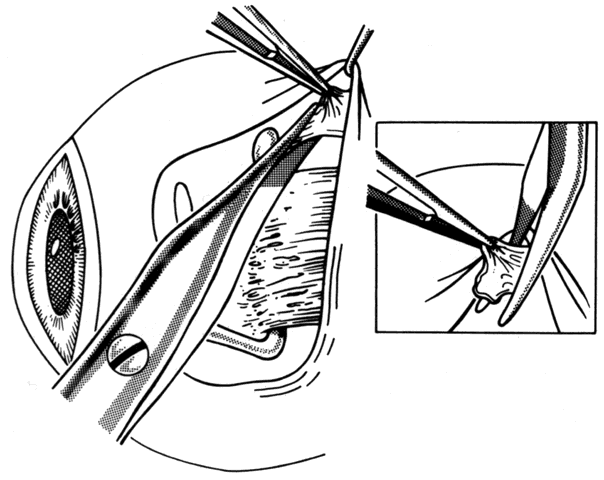
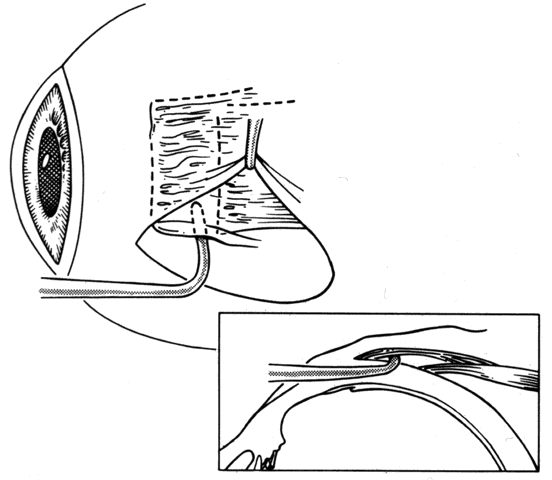
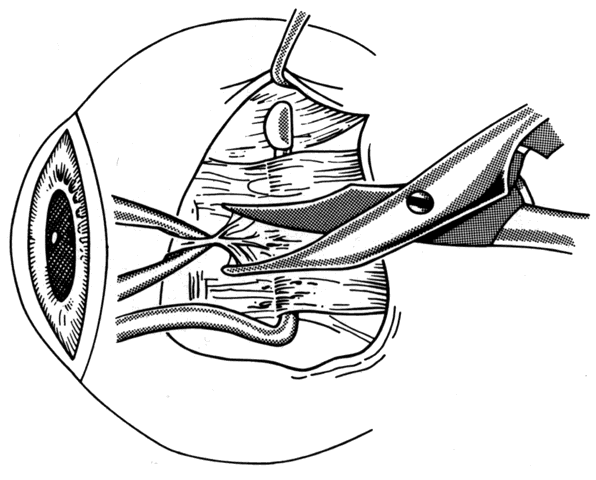
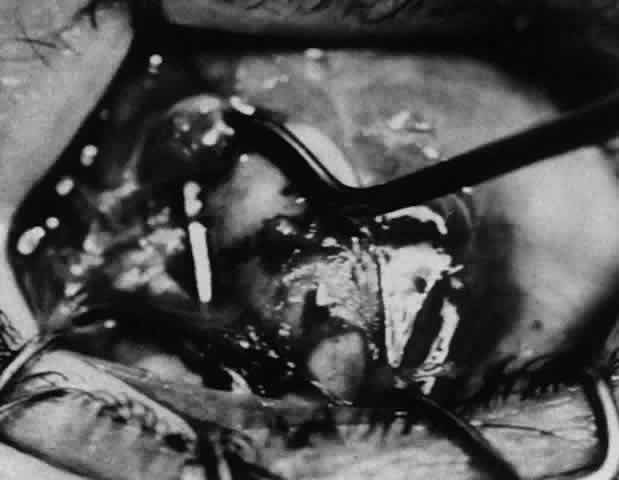
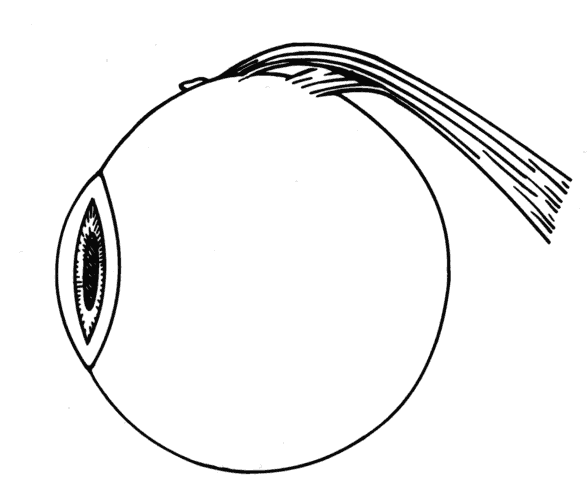
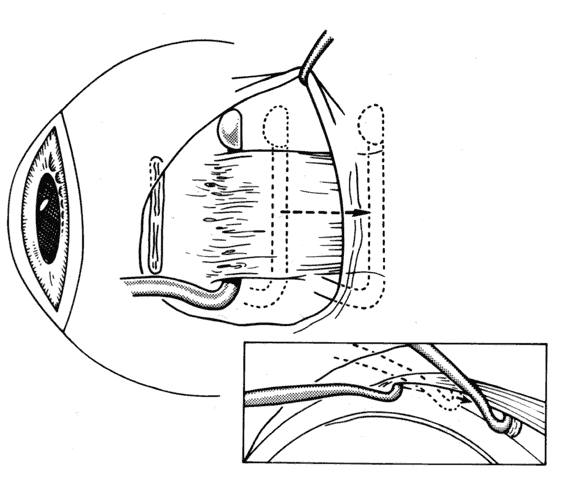
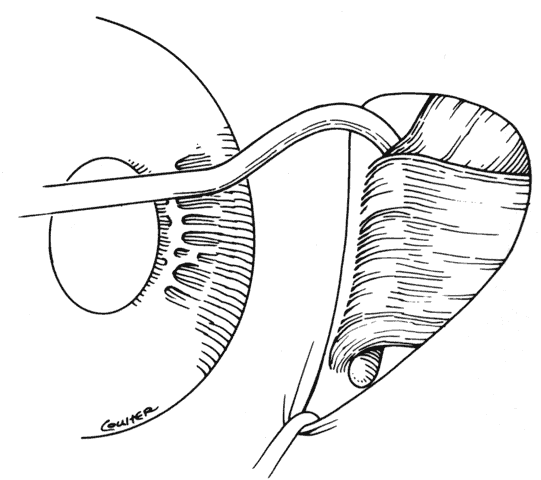
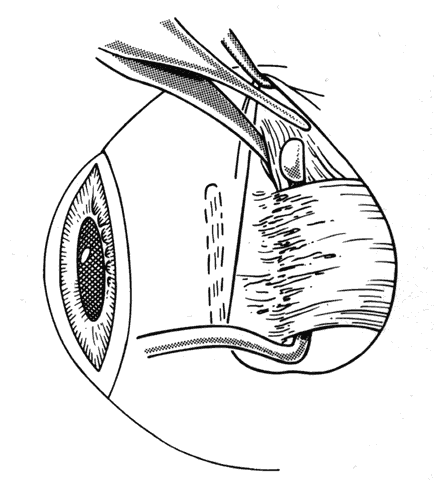
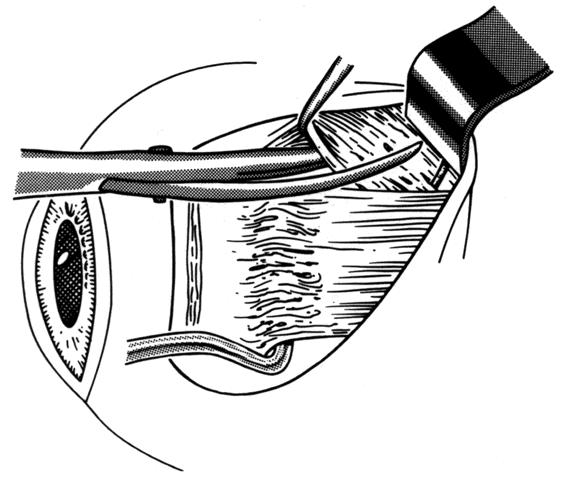
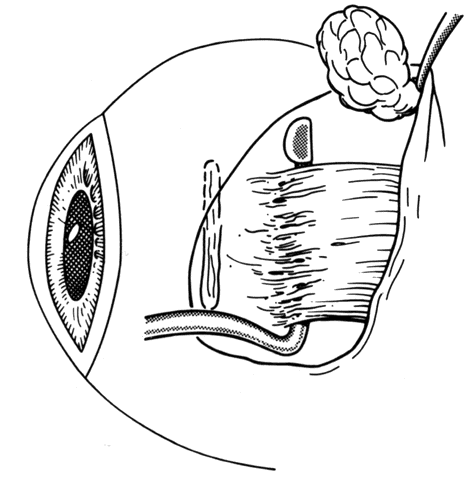
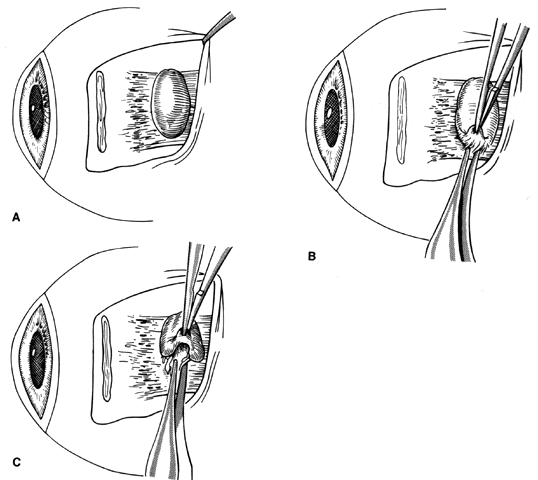
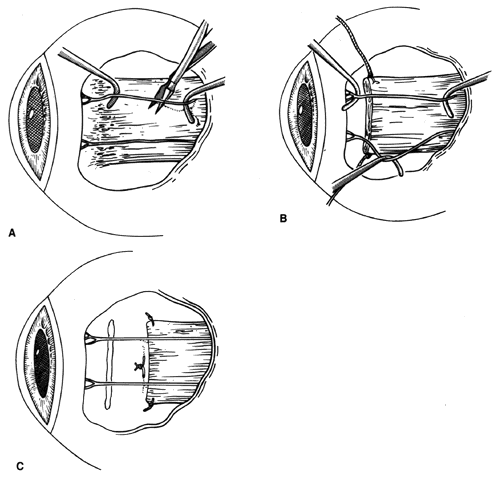
1. Helveston EM: Reoperations in strabismus. Ophthalmology 86:1379, 1979 2. Cooper EL: The surgical management of secondary exotropia. Trans Am Acad Ophthalmol Otolaryngol 65:598, 1961 3. Parks MM: Causes of the adhesive syndrome. In: Symposium on Strabismus. Transactions
of the New Orleans Academy of Ophthalmology, p 269. St. Louis, CV
Mosby, 1978 4. Jampolsky A: Strabismus reoperation techniques. Trans Am Acad Ophthalmol Otolaryngol 79:704, 1971 5. Jampolsky A: Surgical leashes and reverse leashes in strabismus surgical
management. In: Symposium on Strabismus. Transactions of the New Orleans
Academy of Ophthalmology, p 244. St. Louis, CV Mosby, 1978 6. Hermann JS: Masked bilateral superior oblique paresis. J Pediatr Ophthalmol Strabismus 18:43, 1981 7. Raab EL, Parks MM: Recession of the lateral recti: early and late postoperative alignment. Arch Ophthalmol 82:203, 1969 8. Knapp PK: The surgical treatment of persistent horizontal strabismus. Trans Am Ophthalmol Soc 63:75, 1965 9. Metz HS: Restrictive factors in strabismus. Surv Ophthalmol 28:71, 1983 10. Eggers HM, Knapp P: Management of reoperations in strabismus surgery. Int Ophthalmol Clin 25:161, 1985 11. Knapp P: The treatment of persistent horizontal strabismus. Trans Am Ophthalmol Soc 63:75, 1965 12. Pratt-Johnson JA: Fusion and suppression: Development and loss. J Pediatr Ophthalmol Strabismus 29:4, 1992 13. Roper-Hall G, Feibel RM: Measurement of the field of binocular single vision in the evaluation of
incomitant paralytic strabismus. Am Orthoptic J 24:77, 1994 14. Sullivan TJ, Kraft SP, Burach C et al: A functional scoring method for the field of binocular single vision. Ophthalmology 99:575, 1992 15. Repka MX, Arnoldi KA: Lateral incomitance in exotropia: Fact or artifact. J Pediatr Ophthalmol Strabismus 28:125, 1991 16. Guyton DL: Clinical assessment of ocular torsion. Am Orthoptic J 33:7, 1983 17. Locastro AJ, Kim SJ, Biglan AW: Treatment of cyclodiplopia with the Harada-Ito operation. Am Orthoptic J 42:111, 1992 18. Biglan AW, Burnstine RA, Rogers GL et al: Management of strabismus with botulinum A toxin. Ophthalmology 96:935, 1989 19. Rosenbaum AC, Kushner BJ, Kirschen D: Vertical rectus muscle transposition and botulinum toxin (Oculinum) to
medial rectus for abducens palsy. Arch Ophthalmol 107:820, 1989 20. Kittleman WT, Mazow ML: Reoperation in esotropia surgery. Am J Ophthalmol 18:174, 1986 21. King RA, Calhoun JH, Nelson LB: Reoperations for esotropia. J Pediatr Ophthalmol Strabismus 24:136, 1987 22. Biedner B, Yassur Y, David R: Medial rectus re-recession in undercorrected esotropia. J Pediatr Ophthalmol Strabismus 29:89, 1992 23. Lockhart LB, Biglan AW, Hiles DA: A comparison of the effectiveness of bilateral resection to unilateral
marginal myotomy and resection for correcting residual exodeviations. Am Orthoptic J 36:49, 1986 24. Yassur BB: Effect of resection of lateral rectus muscle in undercorrected esotropia. Ophthalmologica 195:45, 1987 25. von Noorden GK: An alternative to marginal myotomy. Am J Ophthalmol 94:285, 1992 26. de Faber JT, von Noorden GK: Medial rectus muscle marginal myotomy for persistent esotropia. Am J Ophthalmol 112:702, 1991 27. Repka MX, Connett JE, Baker JD et al: Surgery in the Prism Adaptation Study: Accuracy and dose response. J Pediatr Ophthalmol Strabismus 29:150, 1992 28. Hiles DA, Watson BA, Biglan AW: Characteristics of infantile esotropia following early bimedial rectus
recession. Arch Ophthalmol 98:676, 1980 29. Parks MM: The monofixation syndrome. Trans Am Ophthalmol Soc 67:609, 1969 30. Arthur BW, Smith JT, Scott WE: Long-term stability of alignment in the monofixation syndrome. J Pediatr Ophthalmol Strabismus 26:224, 1989 31. Prism Adaptation Study Research Group:
Efficacy of prism adaptation in the surgical management of acquired esotropia.
Arch Ophthalmol
108:1248,
1990 32. Shuchett EP, Hiles DA, Biglan AW et al: Posterior fixation suture operation (Faden operation). Ophthalmic Surg 12:578, 1981 33. Ohtsuki H, Hasebe S, Tadokow Y et al: Advancement of medial rectus muscle to the original insertion for consecutive
exotropia. J Pediatr Ophthalmol Strabismus 30:301, 1993 34. Wilson ME, Hoxie J: Facial asymmetry in superior oblique muscle palsy. J Pediatr Ophthalmol Strabismus 30:315, 1993 35. Guyton DL: Exaggerated traction test for the oblique muscles. Ophthalmology 88:1035, 1981 36. Fishman PH, Repka MX, Green WR et al: A primate model of anterior segment ischemia after strabismus surgery. Ophthalmology 97:456, 1990 37. Helveston EM, Alcorn DM, Ellis FD: Inferior oblique inclusion after lateral rectus surgery. Graefes Arch Clin Exp Ophthalmol 226:102, 1988 38. Schlossman A: Personal communication 39. Kennedy JA: Marginal myotomy of the medial rectus. Arch Ophthalmol 84:625, 1970 40. Krocek SE, Heyde EL, Helveston EM: Quantifying the marginal myotomy. Am J Ophthalmol 70:204, 1970 41. Virdi PS, Hayreh SS: Anterior segment ischemia after recession of various recti. Ophthalmology 94:1258, 1987 42. McKeown CA, Lambert HM, Shore JW: Preservation of the anterior ciliary vessels during extraocular muscle
surgery. Ophthalmology 96:498, 1989 43. Davis JS, Biglan AW: Prospective Investigation of the Effectiveness of
Intraoperative Adjustable Sutures for Correction of Strabismus. Trans
Am Ophthalmol Soc XCII:325, 1994 44. Yaacobi Y, Hamed LM, Koul KS et al: Reduction of postoperative adhesions secondary to strabismus surgery in
rabbits. Ophthalmic Surg 23:123, 1992 45. Cole JG, Cole HG: Recession of the conjunctiva in complicated eye muscle operations. Am J Ophthalmol 53:618, 1962 46. Dunlap EA: Plastic implants in muscle surgery: A study of the possible use of plastic
materials in the management of extraocular motility restrictions. Trans Am Ophthalmol Soc 65:393, 1967 47. Searl SS, Metz HS, Lindahl KJ: The use of sodium hyaluronate as a biologic sleeve in strabismus. Am J Ophthalmol 19:259, 1987 48. Elsas FJ, Gowda C, Urry DW: Synthetic polypeptide sleeve for strabismus surgery. J Pediatr Ophthalmol Strabismus 29:284, 1992 49. Scott AD, Miller JM, Collins CC: Eye muscle prosthesis. J Pediatr Ophthalmol Strabismus 29:216, 1992 |