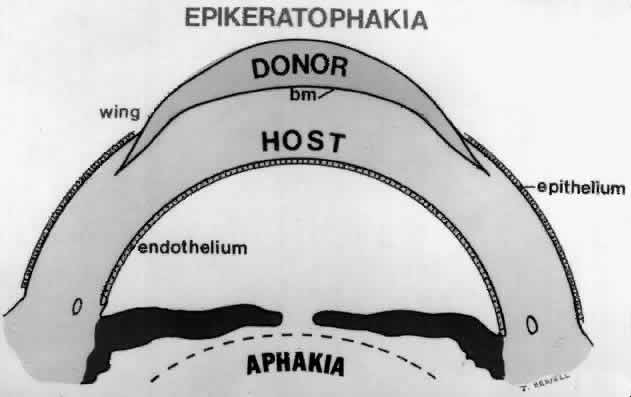
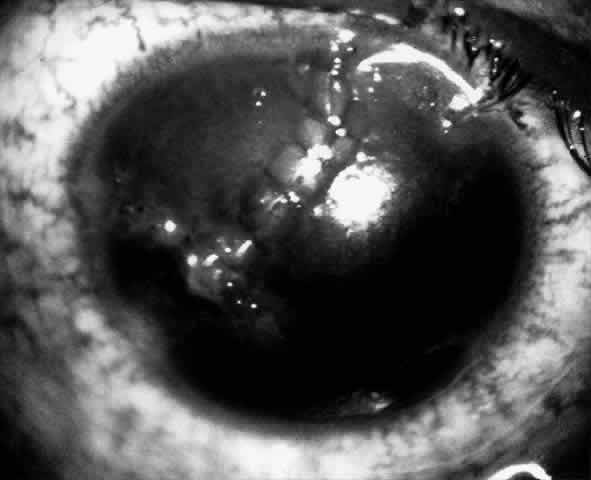
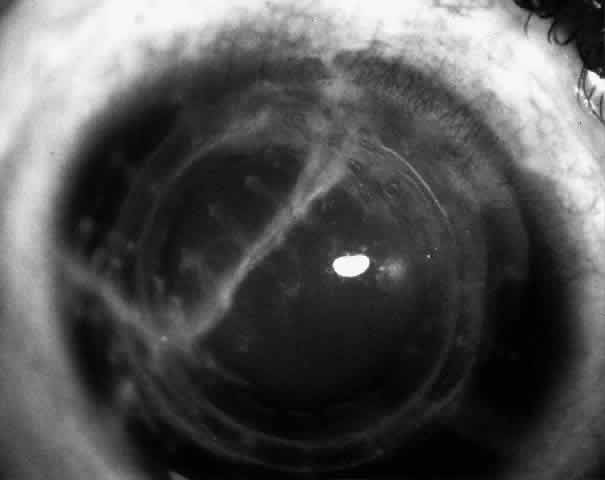
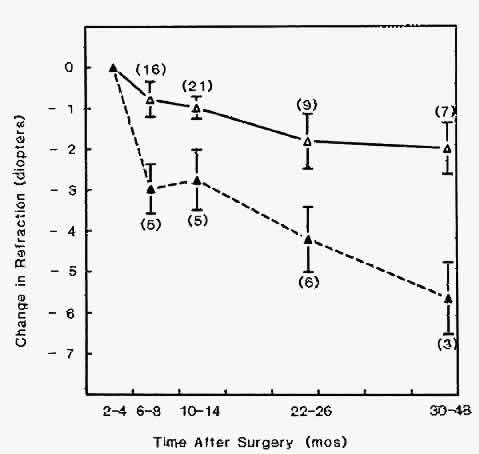
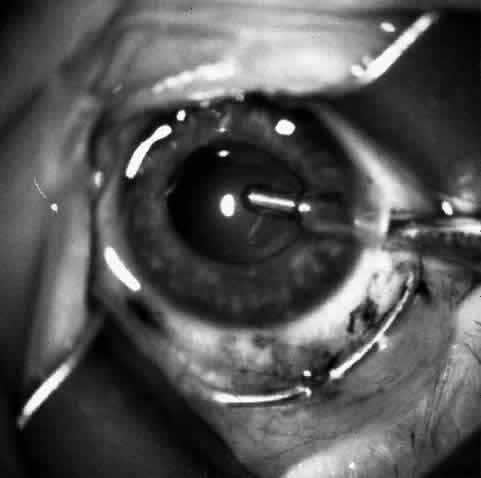
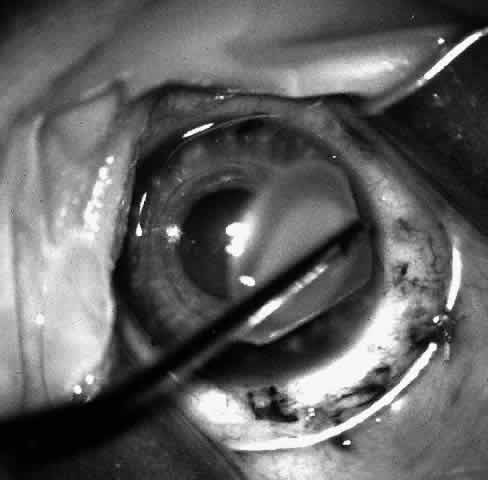
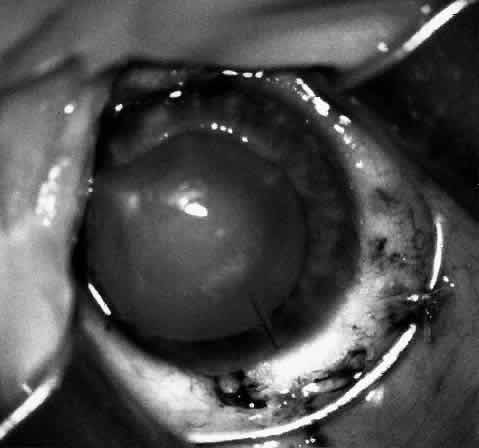
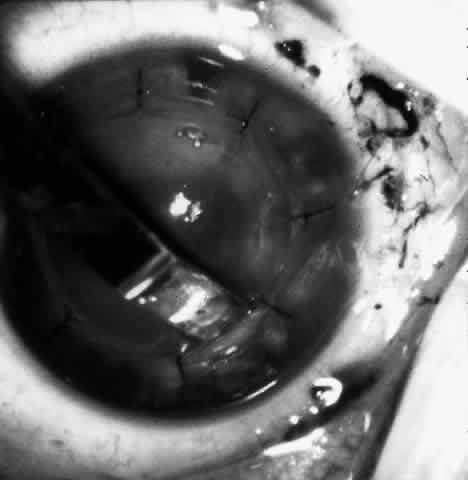
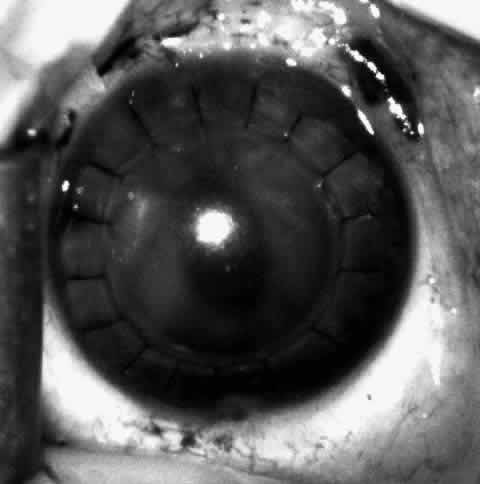
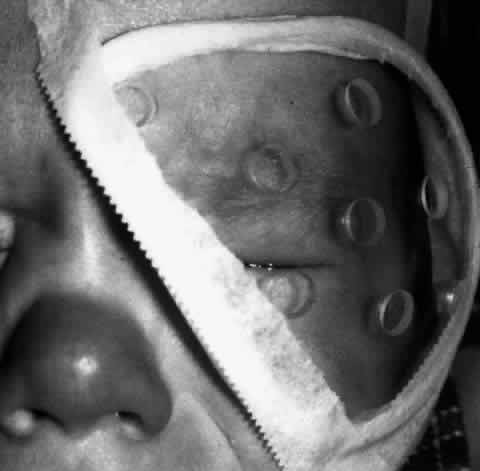
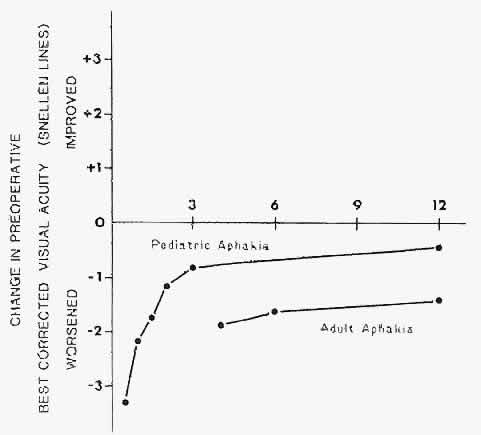
1. Barraquer JI: Keratomileusis and keratophakia. In Rycroft PV (ed): Corneoplastic
Surgery: Proceedings of the 2nd International Corneo-Plastic
Conference, pp 409–443. New York, Pergamon Press, 1969 2. Swinger CA, Krumeich JH, Cassiday D: Planar lamellar refractive keratoplasty. J Refract Surg 2: 17, 1986 3. Krumeich JH, Swinger CA: Nonfreeze epikeratophakia for the correction of myopia. Am J Ophthalmol 103:397, 1987 4. Lieurance RC, Patel AC, Wan WL et al: Excimer laser cut lenticules for epikeratophakia. Am J Ophthalmol 103:475, 1987 5. McDonald MB, Kaufman HE, Aquavella JV et al: The nationwide study of epikeratophakia for aphakia in adults. Am J Ophthalmol 103:358, 1987 6. Morgan KS, McDonald MB, Hiles DA et al: The nationwide study of epikeratophakia for aphakia in children. Am J Ophthalmol 103:366, 1987 7. Morgan KS, McDonald MB, Hiles DA et al: The nationwide study of epikeratophakia for aphakia in older children. Ophthalmology 95:526, 1988 8. McDonald MB, Kaufman HE, Aquavella JV et al: The nationwide study of epikeratophakia for myopia. Am J Ophthalmol 103:375, 1987 9. McDonald MB, Kaufman HE, Durrie DS: Medical Monitors of the Nationwide Epikeratophakia Study: Epikeratophakia
for keratoconus: The nationwide study. Arch Ophthalmol 104: 1294, 1986 10. Morgan KS, Werblin TP, Ashell PA et al: The use of epikeratophakia grafts in pediatric monocular aphakia. J Pediatr Ophthalmol Strabismus 18:23, 1981 11. Morgan KS, Werblin TP, Friedlander MH et al: Epikeratophakia in the pediatric patient: A case report. J Ocul Ther Surg 1(4): 198, 1982 12. Asbell PA, Werblin TP, Loupe DN et al: Secondary surgical procedures after epikeratophakia. Ophthalmic Surg 13:555, 1982 13. Morgan KS, Asbell PA, McDonald MB et al: Preliminary visual results of pediatric epikeratophakia. Arch Ophthalmol 101:1540, 1983 14. Morgan KS, Stephenson GS, McDonald MB et al: Epikeratophakia in children. Ophthalmology 91:780, 1984 15. Arffa RC, Marvelli TL, Morgan KS: Keratometric and refractive results of pediatric epikeratophakia. Arch Ophthalmol 103: 1656, 1985 16. Morgan KS, Stephenson GS: Epikeratophakia in children with corneal lacerations. J Pediatr Ophthalmol Strabismus 22:105, 1985 17. Morgan KS, Marvelli TL, Ellis GS et al: Epikeratophakia in children with traumatic cataracts. J Pediatr Ophthalmol Strabismus 23: 108, 1986 18. Aguirre Vila-Coro A, Goosey JD, Mazow ML et al: Epikeratophakia on pediatric traumatized corneas. J Pediatr Ophthalmol Strabismus 24:182, 1987 19. Morgan KS, Collins CC: Combined cataract extraction and epikeratophakia
in children, J Pediatr Ophthalmol Strabismus 26:14, 1989 20. Cheng KP, Hiles DA, Biglan AW et al: Risk factors for complications following pediatric epikeratoplasty. J Cataract Refract Surg 18:270, 1992 21. Morgan KS, Arffa RC, Marvelli TL et al: Five year followup of epikeratophakia in children. Ophthalmology 93:423, 1986 22. Morgan KS, Braverman DE, Baker JD: The correction of unilateral aphakia in children treated for orbital rhabdomyosarcoma. J Pediatr Ophthalmol Strabismus 27:70, 1990 23. Arffa RC, Donzis PB, Morgan KS et al: Prediction of aphakic refractive error in children. Ophthalmic Surg 18:581, 1987 24. Donzis PB, Kastl PR, Gordon RA: An intraocular lens formula for short, normal, and long eyes. CLAO J 11:95, 1985 25. Hoffer K J: Preoperative evaluation of the cataractous patient. Surv Ophthalmol 29:55, 1984 26. Arffa RC, Marvelli TL, Morgan KS: Long term followup of refractive and keratometric results of pediatric
epikeratophakia. Arch Ophthalmol 104:668, 1986 27. Wagoner MD, Steinert RF: Temporary tarsorrhaphy enhances reepithelialization after epikeratoplasty. Arch Ophthalmol 106:13, 1988 28. Morgan KS, Beuerman RW: Interface opacities in epikeratophakia. Arch Ophthalmol 104: 1505, 1986 |