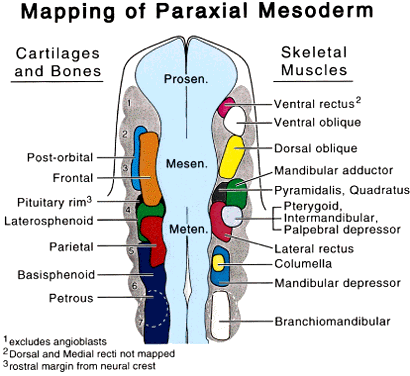
The body axis appears with the genesis of the node and primitive streak. HNF3 β, originally identified as a liver-specific transcription factor, is first found in the node, where it is required for proper morphogenesis of the primitive streak and notochord. In HNF3 β null mutants, anteroposterior patterning persists, but there is no node or notochord formation, and dorsoventral patterning is disturbed. Null embryos have abnormal streaks and affected foregut formation.14 The region just anterior to the node is called the “prechordal plate.” Mesoderm, largely responsible for dorsoventral and anteroposterior patterning of the body axis,15,16 also underlies the future eye-forming part of the prechordal plate, or “eye field,” before neural tube closure. This formation of mesoderm at the prechordal plate and its equivalents, which form the prosencephalon and diencephalon and related head structures will be discussed. Patterning of the prechordal plate differs substantially from that of the rest of the head and trunk. Caudal to the node there is clear rostrocaudal organization that is maintained by several genes. The Hox family of genes is the one best characterized.17 Development of most left-right body features follows this segmental plan, but the prosencephalon is not a part of this organization. It forms as a single, uninterrupted crescent-shaped area extending forward of the node, with no primitive streak. However, interruption of expression in the most anterior of the segmentally organized genes can have a direct impact on the anterior head segment. For example, the homeobox genes XLim1 (and its mammalian homologue Lim1) and KROX 20 are essential for establishment of anterior head structures. Both Lim-/- and KROX 20-/- mutants are truncated just rostral to rhombomere 3, in the metencephalon.18
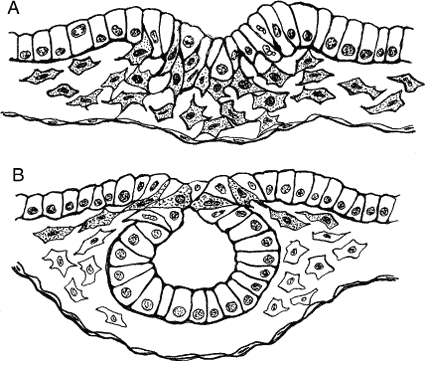
Mesoderm is generated in the prechordal region, as elsewhere, by way of a process known as epithelial-mesenchymal transformation (Fig. 1A), through which epithelial cells lose their tight junctions and move between openings in the underlying basement membrane. Mesenchyme is a uniquely embryonic connective tissue best characterized by the absence of tight junctions between its highly mobile cells and the heterogeneous nature of its extracellular matrix (ECM). Mesenchymal cells advance by “pulling” themselves through the filaments of the ECM, a process that depends on the presence of certain cell adhesion-specific molecules in the ECM and complementary matrix adhesion-specific molecules on the cell surfaces. This has been demonstrated both in vitro and in vivo. For instance, when ectodermal explants are treated with heparinase to remove glycosaminoglycans, morphogenetic movements are inhibited and no mesoderm is formed.19
Mesoderm may be restricted, as opposed to induced, through the action of molecules such as bone morphogenetic proteins (BMPs), which are members of the transforming growth factor- β superfamily of signaling molecules. These molecules are important during the early fate-mapping process,20 although subsequently they may assume different signaling roles. BMP 3 inhibits noggin and goosecoid, thus restricting a subset of mesoderm contributing to the head and anterior trunk (“dorsal mesoderm”).21 Maternally derived BMP 2 promotes generalized mesoderm formation,22 whereas BMP 4 is more specific, inhibiting production of “dorsal” mesoderm and promoting differentiation of “ventral” structures, including the blood, pronephros, and visceral tissues.4
Ventral mesoderm induction can be regulated in part by suppression of the BMP proteins. An example is the recently characterized gene XIPOU2,23 which suppresses activation of goosecoid and XLim1 by activin, thus in turn suppressing BMP 4. If XIPOU2 is injected into the anterior pole of Xenopus blastulas, it reduces the eye field (as measured by Pax-6 gene expression), and the resultant tadpoles exhibit microphthalmia. The normal phenotype can be rescued by chordin. However, injection of XIPOU2 into the posterior vegetal pole produces axis duplication, two heads, and four eyes. This demonstrates the site-specificity of action of a given gene product, another feature that continues to change with development.
The formation of mesoderm from transformed ectodermal cells completes the three primary germ layers; ectoderm, mesenchymal mesoderm, and endoderm. Next, many important lineage commitments relative to the eye and periocular tissues become established, the body axis becomes more fully defined, and dorsoventral, mediolateral spatial coordinates are becoming established through molecular signaling. One of the most important signals involved in axis definition is sonic hedgehog (SHH), which is the mammalian homologue of the Drosophila hedgehog gene. Until recently, it was known that the SHH protein was expressed along the body axis and the posterior margins of limb buds in ways that suggested that the role of this protein involved axial patterning. The SHH knockout mouse demonstrates a role in defining the midline of the body, reinforcing bilaterality, and promoting limb morphogenesis.24 The null phenotype is lethal and includes incompletely fused neural tubes with poorly defined ventral midlines, and severe cyclopia, expressed as one eye in a single frontal socket, with an overlying proboscis and no olfactory placode.
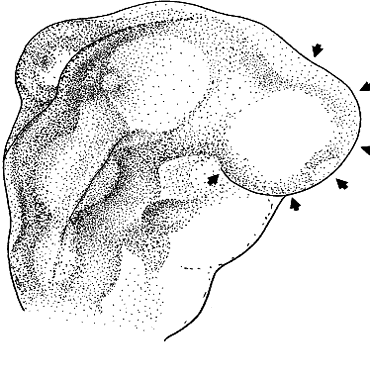
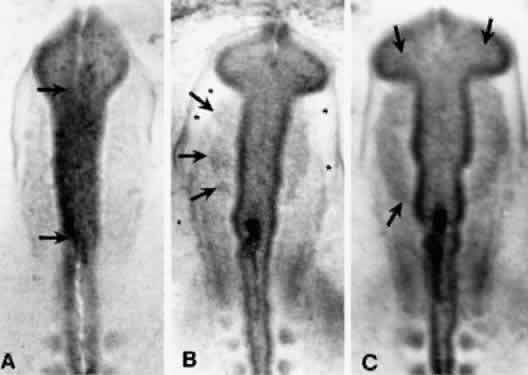
Cyclopia results from incomplete bilateralization of the eye field, which is initially located in the prechordal plate (Fig. 2; also, see elsewhere in these volumes). When the prechordal mesoderm underlying the eye field of Xenopus embryos is removed, only one retina is formed.25 Part of the future prosencephalon, which later forms both the telencephalon and diencephalon, is set aside to form the eyes. This single crescent, or eye field, arises rostral to the primitive streak. The eye field expresses a gene, known as Pax-6, which is essential for eye formation. Transplantation experiments show that pieces of the prechordal plate are able to suppress Pax-6 expression in the retina, but expression of Pax-6 in the prechordal ectoderm is not affected by the presence or location of early optic vesicles.26 This suggests that Pax-6 expression in the prechordal field is spatially segregated into two eye fields through the action of the prechordal mesoderm. Axial mesendoderm positively regulates the medially and ventrally expressed gene Nkx2.1 in the anterior head fold and suppresses laterally and dorsally expressed genes, such as Pax-6. Expression of Nkx2.1 is induced by the prechordal plate.27
|
Human anophthalmia, aniridia, Peters' anomaly, and autosomal dominant keratitis are known to be linked to Pax-6 deficiencies.28–31 In the Pax-6-deficient mouse Sey-sey, eyeless homozygote dysmorphologies include abnormal midline connective tissues,32 perhaps reflecting the default outcome of prechordal ectoderm in the absence of Pax-6. Pax-6 is progressively downregulated until it is restricted to the lens and olfactory placodes. In Sey-sey homozygote mice that fail to form placodes, optic vesicles are formed, but involute.33 Perhaps the ultimate role of Pax-6 in eye formation is most dramatically demonstrated in Drosophila, in which artificial upregulation of the Pax-6 homologue eyeless results in ectopic eye formation all over the body, including the legs, antennae, and wings!34 This startling result, coupled with the prevalence of Pax-6 homologues among species, led the authors to suggest that Pax-6 may be a “master control gene” for eye morphogenesis. It also demonstrates that the general functions of many key regulatory genes have been conserved through thousands of milleniums of animal evolution.
Usually, teratogenic agents are blamed for failure of the eye field to separate. For some years, veterinarians have been aware that pregnant ewes grazing on fields containing specimens of the weed Veratrum give birth to cyclopic lambs if they eat the plant during the first weeks of gestation.35 Earlier in this century, studies on eye formation led to the discovery that fish embryos exposed to magnesium in sufficient concentrations developed cyclopia.36 The percentage of larvae with cyclopia was dependent on the amount of magnesium in the water, whereas the severity of the phenotype (ranging from close-set eyes, to two eyes in one frontal orbit, to a true cyclops with one eye in a frontal socket) was determined by the length of the exposure. The most common teratogen affecting human births is ethanol, which disrupts the prosencephalon by limiting its size; the affected child may show microphthalmia, short palpebral fissures, deficiencies of the philtral region, and a long upper lip.37 The otherwise low incidence of cyclopia in nature suggests that chemical agents with the potential to cause major prosencephalic defects are not generally encountered, and that the window of development during which the embryo is vulnerable is short.
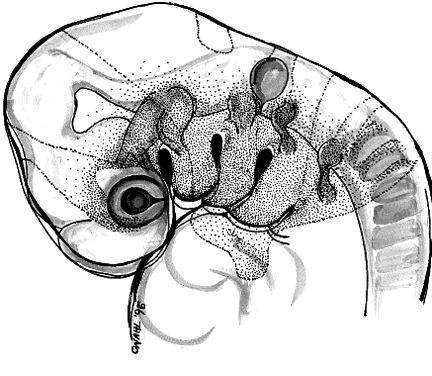
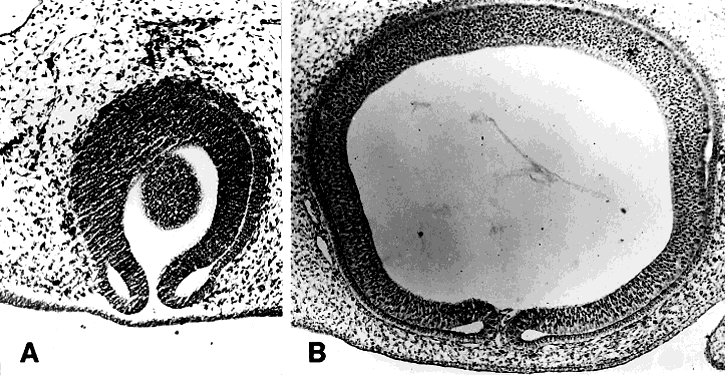
The periocular area undergoes marked changes in tissue relations as the brain grows. As the flat neural tube expands mediolaterally, its walls form paired lateral outpockets called optic vesicles, which later become the optic cups (Fig. 3). The prechordal and paraxial mesoderm originally underlying the rostral neural plate expands laterally and rostrocaudally in congruence with the neural tissue until the evagination of the optic vesicles pushes adjacent mesoderm caudally and ventrally. This leaves the optic vesicle devoid of an enveloping mesenchymal layer except on its caudal (future temporal) surface. The remaining mesenchymal space posterior to these rudimentary optic vesicles is characterized simply as “paraxial mesoderm.” This mesenchyme is not displaced or migratory but consists of a uniform population of mesodermal, stellate cells located almost exclusively in the dorsal and ventrolateral (12 to 6 o'clock) positions. The remaining periocular area is acellular, consisting solely of ECM in the space between the optic vesicle and overlying ectoderm.
Thus, the neural epithelium of the optic vesicle is in direct apposition to the surface ectoderm along its lateral and anterior surfaces. Normal development of the eye depends on the absence of mesenchyme in these areas, as has been demonstrated by the mouse mutant extra-toes, in which invasion of mesenchymal cells along the lateral margin prevents normal formation of the lens.38 In normal animals, mesenchyme that may become trapped between the invaginating optic vesicle and thickening lens undergoes necrosis and is resorbed. In a congenitally anophthalmic mouse strain, this trapped mesenchymal population failed to undergo apoptosis, and the normal sequence of necrotic loci in the developing eye was lacking.39
It is the failure of the lens placode to contact the optic vesicle as a result of inappropriately placed mesenchyme that results in anophthalmia among these genetically defective animals.33,40 The lens placode also plays a key role in ocular expansion, perhaps related to generation of the vitreous. Growth of the retina is unaffected by eye size, because the retina will continue to proliferate beyond the confines of a miniaturized eye. Mesenchymal processes that lead to expansion of the globe do not control or otherwise affect the mitotic rate within the retina. Retinal convolutions may fill the eyecups of microphthalmic or “anophthalmic” animals, a phenomenon that can be duplicated experimentally by the use of teratogens.41