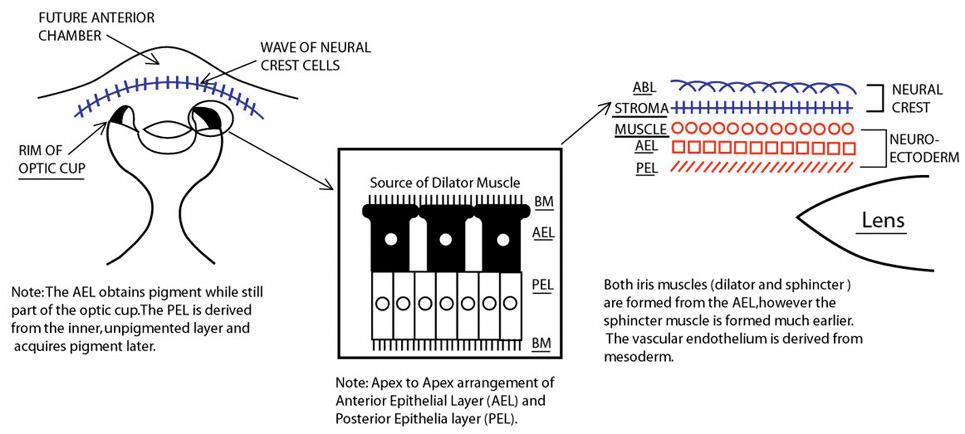
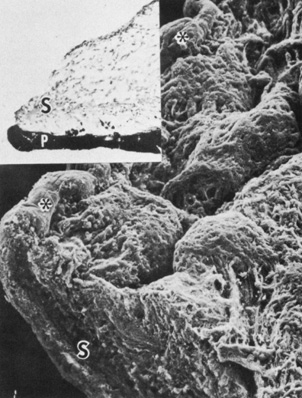
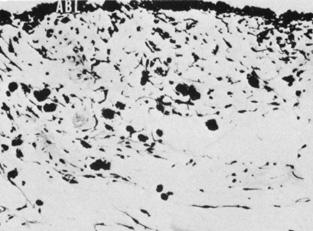
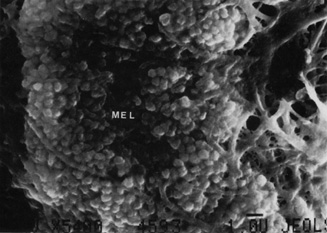
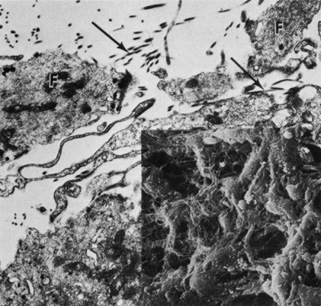
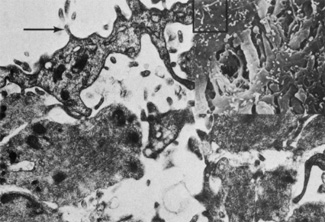
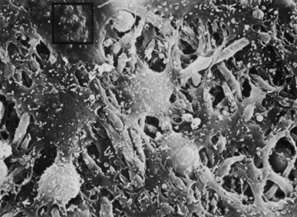
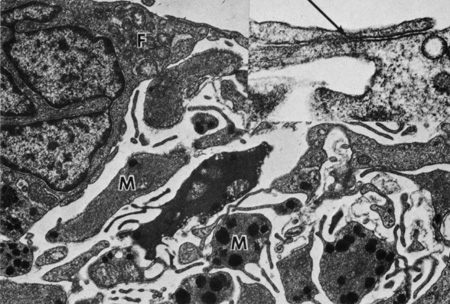
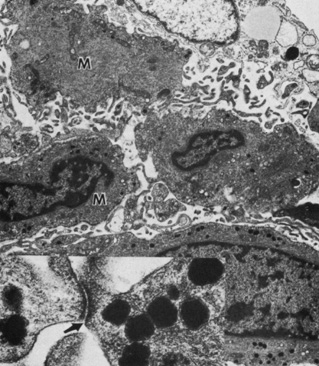
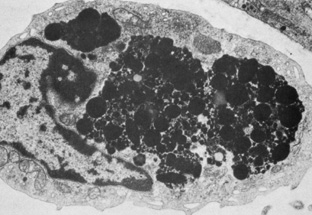
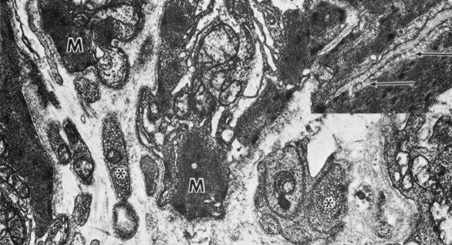
Embryologically, the structure of the iris can be divided into two different developmental layers: iris pigment epithelium (IPE) of neuroectodermal origin and iris stroma of neural crest origin. The cells of the IPE arise from mesoderm, and the stromal blood vessels begin as penetrating branches from the developing vascular network of the ciliary body (Fig. 1). Although data regarding the origin of both iris muscles (sphincter and dilator) are incomplete, they likely arise from neuroectoderm.4,6
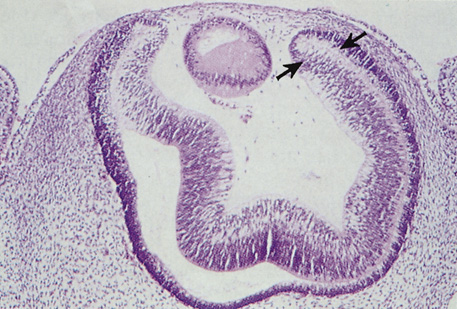
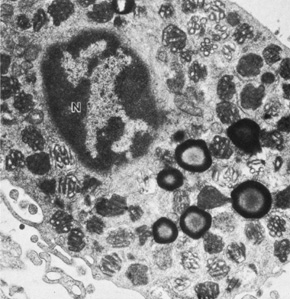
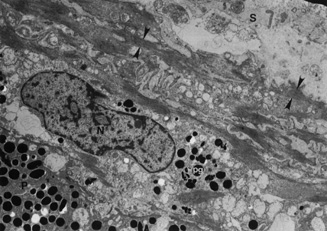
The embryologic precursors of the iris are first visible at six weeks gestational age. The neural ectodermal derivatives (iris epithelium and muscle) arise from the anterior rim of the optic cup. At about six weeks gestational age, these cells begin to proliferate rapidly and extend anteriorly.10 During development, an apparent dilation, called the marginal sinus of von Szily, can be seen at the tip of the optic cup between the two layers of epithelium. Although this sinus is now thought to represent an artifact, the two layers of neuroepithelium become functionally divided into the anterior and posterior pigment epithelium (Fig. 2).11 Embryologically, the anterior epithelium becomes pigmented while still part of the optic cup; however, the pigmentation of the posterior epithelium (derived from the inner, unpigmented layer of the optic cup) occurs later, proceeding toward the ciliary region, and is completed by seven months gestation.12
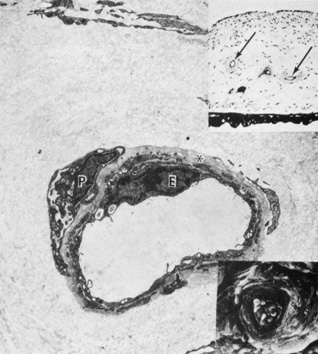
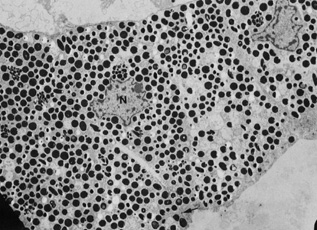
Differentiation of the iris stromal elements begins slightly earlier than iris neuroepithelial growth and proceeds as a wave of mesenchymal migration at about six weeks' gestation. This forms the anlage of iris stroma, a continuous papillary membrane, and the tunica vasculosa lentis (a transient vascular network that supplies the developing lens). Iris stromal pigmentation does not occur until after 24 weeks. The developing iris lacks a pupil because the original stroma is a continuous sheet of tissue with epithelial cells growing inward, toward the center of the iris.4,11
The vascular framework of the iris also begins at six weeks as a blind axial outgrowth from the annular vessels in the mesenchyme associated with the rim of the optic cup and forms the anterior part of the tunica vasculosa lentis (lamina iridopupillaris). By the third month, branches of the nasal and the temporal long posterior ciliary arteries unite with peripheral vessels of the lamina iridopupillaris to form the major arterial circle. The pupil is created by cellular and vascular remodeling of the iridopupillary membrane during the fifth month of gestation.4,11
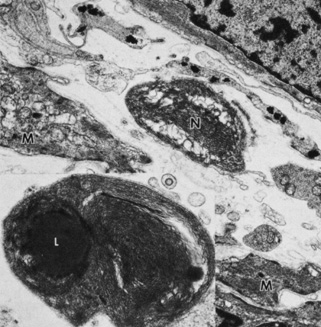
The development of the iris sphincter muscle first appears as basal infoldings in the cells of the anterior layer of neuroepithelium and can be seen by light microscopy at about eleven weeks. By the fifth month, myofibrils begin to be synthesized by the developing tissue; the muscle comes to lie free in the posterior mesenchymal layer by the eighth month of gestation. The formation of the dilator muscle fibers occurs later and is first identified in the sixth month as fine fibrils in the anterior iris epithelium.
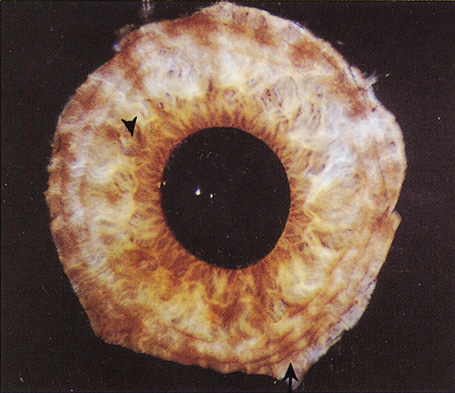
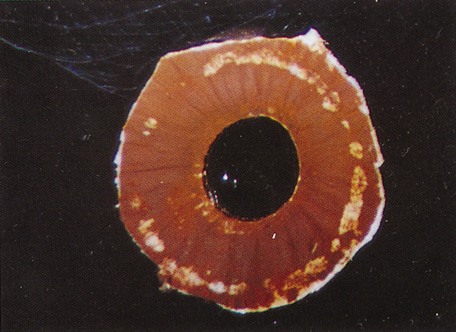
The iris is near its adult size at birth. The most dynamic changes occur in the first few postnatal years and involve the color of the iris and the extracellular matrix. Embryologically, the anterior epithelium becomes pigmented while still part of the optic cup; however, the pigmentation of the posterior epithelium (derived from the inner, unpigmented layer of the optic cup) occurs later, proceeding toward the ciliary region, and is completed by seven months' gestation. Postnatal changes in iris color occur by the gradual accumulation of melanin in the stroma and anterior border layer as discussed later.