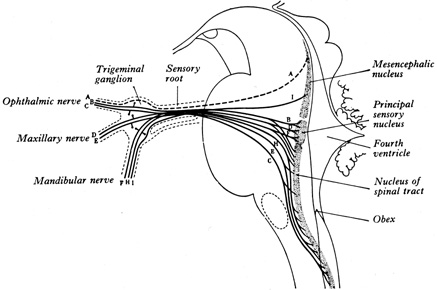
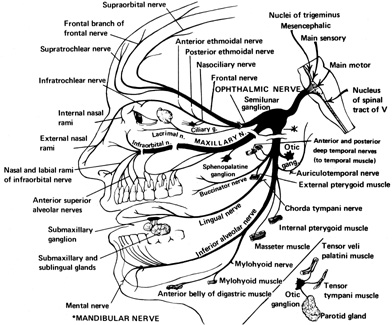
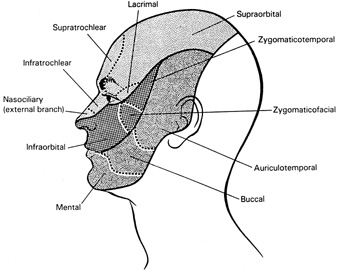
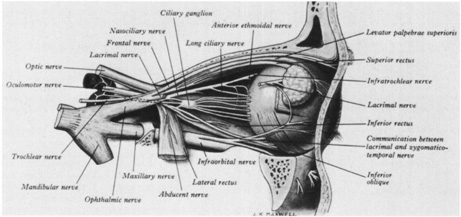
1. Burton H: Somatosensory sensations from the eye. In Moses RA (ed.): Adler's Physiology of the Eye. St. Louis: CV Mosby, 1981:63–68 2. Ray BS, Wolff HG: Experimental studies on headache. Pain-sensitive structures of the
head and their significance in headache. Arch Surg 41:813, 1940 3. Feindel W, Penfield W, McNaughton F: The tentorial nerves and localization of intracranial pain in man. Neurology 10:555, 1960 4. Wirth FP, Van Buren JM: Referral of pain from dural stimulation in man. J Neurosurg 34:630, 1971 5. Moonie GT, Rees DL, Elton D: The oculocardiac reflex during strabismus surgery. Can Anaesth Soc J 11:621, 1964 6. Lang S, Lanigan DT, van der Wal M: Trigeminocardiac reflexes: maxillary and mandibular variants of the oculocardiac
reflex. Can J Anaesth 38:757, 1991 7. Barnard NA, Bainton R: Bradycardia and the trigeminal nerve. J Craniomaxillofac Surg 18:359, 1990 8. Haerer AF: DeJong's The Neurologic Examination, 5th ed. Philadelphia: JB Lippincott, 1992:165–179 9. Kimura J: The blink reflex. In Kimura J: Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice, 2nd ed. Philadelphia: FA Davis, 1989:307–331 10. Ongerboer de Visser BW, Melchelse K, Megens PHA: Corneal reflex latency in trigeminal nerve lesions. Neurology 27:1164, 1977 11. Kimura J: Somatosensory and motor evoked potentials. In Kimura J: Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice, 2nd ed. Philadelphia: FA Davis, 1989:395–397 12. Kimura J: H, T, masseter and other reflexes. In Kimura J: Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice, 2nd ed. Philadelphia: FA Davis, 1989:361–363 13. Goor C, Ongerboer de Visser BW: Jaw and blink reflexes in trigeminal nerve lesions: an electrodiagnostic
study. Neurology 26:95, 1976 14. Taverner D: The localization of isolated cranial nerve lesions. In Vinken PJ, Bruyn GW (eds.): Handbook of Clinical Neurology, Vol. 2. Amsterdam: North-Holland, 1969:54–59 15. Martin XY, Safran AB: Corneal hypoesthesia. Surv Ophthalmol 33:28, 1988 16. De Haas EBH: Desiccation of cornea and conjunctiva after sensory denervation. Arch Ophthalmol 67:439, 1962 17. Mackie IA: Role of the corneal nerves in destructive diseases of the cornea. Trans Ophthalmol Soc UK 98:343, 1978 18. Duke-Elder S, Leigh AG: Diseases of the outer eye. In Duke-Elder S (ed.): System of Ophthalmology, Vol. 8. St. Louis: CV Mosby, 1965:809 19. Rowbotham GF: Acute Injuries of the Head. Baltimore: Williams & Wilkins, 1964:422–424 20. Goldstein NP, Gibilisco JA, Rushton JG: Trigeminal neuropathy and neuritis: a study of etiology with emphasis on
dental causes. JAMA 184:458, 1963 21. Ferdousi AM, MacGregor AJ: The response of the peripheral branches of the trigeminal nerve to trauma. Int J Oral Surg 14:41, 1985 22. Rose GE, Wright JE: Trigeminal sensory loss in orbital disease. Br J Ophthalmol 78:427, 1994 23. Selby G: Diseases of the fifth cranial nerve. In Dyck PJ, Thomas PK, Lambert EH, Bunge R (eds.): Peripheral Neuropathy, 2nd ed., Vol. II. Philadelphia: WB Saunders, 1984:1224–1265 24. Blau JN, Harris M, Kennett S: Trigeminal sensory neuropathy. N Engl J Med 281:873, 1969 25. Peñarrocha M, Alfaro A, Bagán JV, et al: Idiopathic trigeminal sensory neuropathy. J Oral Maxillofac Surg 50:472, 1992 26. Horowitz SH: Isolated facial numbness: clinical significance and relation to trigeminal
neuropathy. Ann Intern Med 80:49, 1974 27. Spillane JD, Wells CEC: Isolated trigeminal neuropathy: a report of 16 cases. Brain 82:391, 1959 28. Hutchins LG, Harnsberger HR, Hardin CW, et al: The radiologic assessment of trigeminal neuropathy. AJR Am J Roentgenol 153:1275, 1989 29. Rorick MB, Chandar K, Colombi BJ: Inflammatory trigeminal sensory neuropathy mimicking trigeminal neurinoma. Neurology 46:1455, 1996 30. Cruccu G, Inghilleri M, Berardelli A, et al: Pathophysiology of hemimasticatory spasm. J Neurol Neurosurg Psychiatry 57:43, 1994 31. Thompson PD, Carroll WM: Hemimasticatory spasm—a peripheral paroxysmal cranial neuropathy? J Neurol Neurosurg Psychiatry 46:274, 1983 32. Trobe JD, Glaser JS, Post JD: Meningiomas and aneurysms of the cavernous sinus: neuro-ophthalmologic
features. Arch Ophthalmol 96:457, 1978 33. Inoue T, Fukui M, Matsushima T, et al: Neurinoma in the cavernous sinus: report of two cases. Neurosurgery 27:986, 1990 34. Tolosa E: Periarteritic lesions of the carotid siphon with the clinical features
of a carotid infraclinoidal aneurysm. J Neurol Neurosurg Psychiatry 17:300, 1954 35. Hunt WE, Meagher JN, LeFever HE, et al: Painful ophthalmoplegia: its relation to indolent inflammation of the cavernous
sinus. Neurology 11:56, 1961 36. Juncos JL, Beal MF: Idiopathic cranial polyneuropathy: a fifteen-year experience. Brain 110:197, 1987 37. Spector RH, Schwartzman RJ: Benign trigeminal and facial neuropathy. Arch Intern Med 135:992, 1975 38. Raeder JG: Paratrigeminal paralysis of oculo-pupillary sympathetics. Brain 47:149, 1924 39. Marsh RJ, Cooper M: Ophthalmic herpes zoster. Eye 7:350, 1993 40. Tomkinson A, Roblin DG, Brown MJKM: Hutchinson's sign and its importance in rhinology. Rhinology 33:180, 1995 41. Roberts TV, Francis IC, Kappagoda MB, et al: Herpes zoster chorioretinopathy. Eye 9:594, 1995 42. Smith EF, Santamarina L, Wolintz AH: Herpes zoster ophthalmicus as a cause of Horner syndrome. J Clin Neuroophthalmol 13:250, 1993 43. Acers TE: Herpes zoster ophthalmicus with contralateral hemiplegia. Arch Ophthalmol 71:371, 1964 44. Kuroiwa Y, Furukawa T: Hemispheric infarction after herpes zoster ophthalmicus: Computed tomography
and angiography. Neurology 31:1030, 1981 45. NcNeil JD, Horowitz M: Contralateral hemiplegia complicating herpes zoster ophthalmicus. J R Soc Med 84:501, 1991 46. Severson EA, Baratz KH, Hodge DO, et al: Herpes zoster ophthalmicus in Olmsted County, Minnesota: have systemic
antivirals made a difference. Arch Ophthalmol 121:386, 2003 47. Tyring S, Engst R, Corriveau C, et al: Famciclovir for ophthalmic zoster: a randomized aciclovir controlled study. Br J Ophthalmol. 85:576, 2001 48. Colin J, Prisant O, Cochener B, et al: Comparison of the efficacy and safety of valaciclovir and acyclovir for
the treatment of herpes zoster ophthalmicus. Ophthalmology 107:1507, 2000 49. Kost RG, Straus SE: Postherpetic neuralgia—pathogenesis, treatment, and prevention. N Engl J Med 335:32, 1996 50. Gradenigo G: A special syndrome of endocranial otitic complications (paralysis
of the motor oculi externus of otitic origin). Ann Otol 13:637, 1904 51. Tutunuoglu S, Uran N, Kavas I, et al: Gradenigo syndrome: a case report. Pediatr Radiol 23:556, 1993 52. Hedeman LS, Lewinsky BS, Lochridge GK, et al: Primary malignant schwannoma of the gasserian ganglion: report of two cases. J Neurosurg 48:279, 1978 53. Tancioni F, Gaetani P, Villani L, et al: Neurinoma of the trigeminal root and atypical trigeminal neuralgia: case
report and review of the literature. Surg Neurol 44:36, 1995 54. Summers CG, Wirtschafter JD: Bilateral trigeminal and abducens neuropathies following low-velocity, crushing
head injury. J Neurosurg 50:508, 1979 55. Maurice-Williams RS, Pilling J: Trigeminai sensory symptoms associated with hydrocephalus. J Neurol Neurosurg Psychiatry 40:641, 1977 56. Hooge JP, Redekop WK: Trigeminal neuralgia in multiple sclerosis. Neurology 45:1294, 1995 57. Olafson RA, Rushton JG, Sayre GP: Trigeminal neuralgia in a patient with multiple sclerosis: an autopsy report. J Neurosurg 24:755, 1966 58. Currier RD, Giles CL, DeJong RN: Some comments on Wallenberg's lateral medullary syndrome. Neurology 11:778, 1961 59. Meige H: Les convulsions de la face, une forme clinique de convulsion faciale, bilaterale
et mediane. Rev Neurol (Paris) 20:437, 1910 60. Marsden CD: Blepharospasm-oromandibular dystonia syndrome (Brueghel's
syndrome): a variant of adult onset torsion dystonia? J Neurol Neurosurg Psychiatry 39:1204, 1976 61. Berardelli A, Rothwell JC, Marsden CD: Pathophysiology of blepharospasm and oromandibular dystonia. Brain 108:593, 1985 62. Jankovic J, Orman J: Botulinum A toxin for cranial-cervical dystonia: a double blind, placebo-controlled
study. Neurology 37:616, 1987 63. Olney RK: AAEM minimonograph No. 38: neuropathies in connective tissue disease. Muscle Nerve 15:531, 1992 64. Walsh FB, Hoyt WF: Clinical Neuro-ophthalmology. Baltimore: Williams & Wilkins, 1969:350–433, 1351–1361 65. Ross AT: Recurrent cranial nerve palsies in diabetes mellitus. Neurology 12:180, 1962 66. Brisman R: Bilateral trigeminal neuralgia. J Neurosurg 67:44, 1987 67. Barker FG, Jannetta PJ, Bissonette DJ, et al: The long-term outcome of microvascular decompression for trigeminal
neuralgia. N Engl J Med 334:1077, 1996 68. Taha JM, Tew JM, Buncher CR: A prospective 15-year follow up of 154 consecutive patients with
trigeminal neuralgia treated by percutaneous stereotactic radiofrequency
thermal rhizotomy. J Neurosurg 83:989, 1995 69. Herzberg L: Familial trigeminal neuralgia. Arch Neurol 37:285, 1980 70. Meaney JFM, Eldridge PR, Dunn LT, et al: Demonstration of neurovascular compression in trigeminal neuralgia with
magnetic resonance imaging. J Neurosurg 83:799, 1995 71. Nomura T, Ikezaki K, Matsushima T, et al: Trigeminal neuralgia: differentiation between intracranial mass lesions
and ordinary vascular compression as causative lesions. Neurosurg Rev 17:51, 1994 72. Dandy WE: Concerning the cause of trigeminal neuralgia. Am J Surg 24:447, 1934 73. Adams CBT, Kaye AH, Teddy PJ: The treatment of trigeminal neuralgia by posterior fossa microsurgery. J Neurol Neurosurg Psychiatry 45:1020, 1982 74. Hamlyn PJ, King TT: Neurovascular compression in trigeminal neuralgia: a clinical and anatomical
study. J Neurosurg 76:948, 1992 75. Jannetta PJ: Microvascular decompression of the trigeminal nerve root entry zone. In Rovit RL, Murali R, Jannetta PJ (eds.): Trigeminal Neuralgia. Baltimore: Williams & Wilkins, 1990:201–222 76. Adams CBT: Microvascular compression: an alternative view and hypothesis. J Neurosurg 57:1, 1989 77. Blom S: Tic douloureux treated with new anticonvulsant. Arch Neurol 9:285, 1963 78. Green MW, Selman JE: Review article: the medical management of trigeminal neuralgia. Headache 31:588, 1991 79. Zakrzewska JM, Patsalos PN: Long-term cohort study comparing medical (oxcarbazepine) and
surgical management of intractable trigeminal neuralgia. Pain 95:259, 2002 80. Iannone A, Baker AB, Morrell F: Dilantin in the treatment of trigeminal neuralgia. Neurology 8:126, 1958 81. Zakrzewska JM, Chaudry Z, Numikko TJ, et al: Lamotrigine (Lamictal) in refractory trigeminal neuralgia: results
from a double-blind placebo controlled crossover trial. Pain 73:223, 1997 82. Reder AT, Arnason BGW: Trigeminal neuralgia in multiple sclerosis relieved by a prostaglandin
E analogue. Neurology 45:1097, 1995 83. Loeser JD: What to do about tic douloureux. JAMA 239:1153, 1978 84. Sweet WH, Wepsic JG: Controlled thermocoagulation of trigeminal ganglion and rootlets for differential
destruction of pain fibers. Part 1: Trigeminal neuralgia. J Neurosurg 39:143, 1974 85. Apfelbaum RI: A comparison of percutaneous radiofrequency trigeminal neurolysis and microvascular
decompression of the trigeminal nerve for the treatment of
tic douloureux. Neurosurgery 1:16, 1977 86. Rovit RL: Percutaneous radiofrequency thermal coagulation of the gasserian ganglion. In Rovit RL, Murali R, Jannetta PJ (eds.): Trigeminal Neuralgia. Baltimore: Williams & Wilkins, 1990:109–136 87. Kondziolka D, Perez B, Flickinger JC, et al: Gamma knife radiosurgery for trigeminal neuralgia: results and expectations. Arch Neurol 55:1524, 1998 88. Smith ZA, DeSalles AAF, Frighetto L, et al: Dedicated linear accelerator radiosurgery for the treatment of trigeminal
neuralgia. J Neurosurg 99:511, 2003 89. Young RF, Vermeulen SS, Grimm P, et al: Gamma knife radiosurgery for treatment of trigeminal neuralgia: idiopathic
and tumor related. Neurology 48:608, 1997 90. Horton BT, MacLean AR, Craig WM: A new syndrome of vascular headache. Results of treatment with histamine: Preliminary
report. Proceedings of the Staff Meetings of the Mayo Clinic 14:257, 1939 91. Kudrow L: The pathogenesis of cluster headache. Curr Opin Neurol 7:278, 1994 92. Turkewitz LJ, Wirth O, Dawson GA, et al: Cluster headache following head injury: a case report and review of the
literature. Headache 32:504, 1992 93. Matharu MS, Boes CJ, Goadsby PJ: Management of trigeminal autonomic cephalgias and hemicrania continua. Drugs 63:May A, Leone M: Update on cluster headache. Curr Opin Neurol 16:333, 2003 94. 1637, 2003 95. Leone M, Franzini A, Broggi G, et al: Hypothalamic deep brain stimulation for intractable chronic cluster headache: a 3-year
follow-up. Neurol Sci 24:S143, 2003 96. Grimson BS, Thompson HS: Raeder's syndrome. A clinical review. Surv Ophthalmol 24:199, 1980 97. Sjaastad O, Elsås T, Shen J, et al: Raeder's syndrome: “anhidrosis,” headache, and a proposal
for a new classification. Funct Neurol 9:215, 1994 98. Kashihara K, Ito H, Yamamoto S, et al: Raeder's syndrome associated with intracranial internal carotid artery
aneurysm. Neurosurgery 20:49, 1987 99. Watanabe K, Tanahashi N, Nara M: Neurosyphilis presenting with Raeder's syndrome. Ann Neurol 25:418, 1989 100. Costen JB: Neuralgias and ear symptoms associated with disturbed function of the temporomandibular
joint. JAMA 107:252, 1936 101. Kirk WS: Chronic temporomandibular joint disease and head pain: response to surgery. N C Med J 54:30, 1993 102. Helgason S, Petursson G, Gudmundsson S, et al: Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective
study with long term follow up. BMJ 321:794, 2000 103. Rowbotham MC, Davies PS, Fields HL: Topical lidocaine gel relieves postherpetic neuralgia. Ann Neurol 37:246, 1995 104. Dubinsky RM, Kabbani H, El-Chami Z, et al: Practice parameter: treatment of postherpetic neuralgia. An evidenced-based
report of the quality standards subcommittee of the American
Academy of Neurology. Neurology 63:959, 2004 105. Rowbotham M, Harden N, Stacey B, et al: Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled
trial. JAMA 280:1837, 1998 106. Loeser JD: Herpes zoster and postherpetic neuralgia. Pain 25:149, 1986 107. Keczkes K, Basheer AM: Do corticosteroids prevent post-herpetic neuralgia? Br J Dermatol 102:551, 1980 108. Whitley RJ, Weiss H, Gnann JW Jr, et al: Acyclovir with and without prednisone for the treatment of herpes zoster. A
randomized, placebo-controlled trial. The National Institute
of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med 125:376, 1996 109. Wood MJ, Johnson RW, McKendrick MW, et al: A randomized trial of acyclovir for 7 days or 21 days with and without
prednisolone for treatment of acute herpes zoster. N Engl J Med 330:896, 1994 110. Jackson JL, Gibbons R, Meyer G, et al: The effect of treating herpes zoster with oral acyclovir in preventing
postherpetic neuralgia. A meta-analysis. Arch Intern Med 157:909, 1997 111. Türp JC, Gobetti JP: Trigeminal neuralgia versus atypical facial pain: a review of the literature
and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81:424, 1996 112. Madland G, Feinmann C: Chronic facial pain: a multidisciplinary problem. J Neurol Neurosurg Psychiatry 71:716, 2001 113. Cusick JF: Atypical trigeminal neuralgia. JAMA 245:2328, 1981 114. Weddington WW, Blazer D: Atypical facial pain and trigeminal neuralgia: a comparison study. Psychosomatics 20:348, 1979 115. Rosenblatt MA, Sakol PJ: Ocular and periocular pain. Otolaryngol Clin North Am 22:1173, 1989 116. Knox DL, Cogan DG: Eye pain and homonymous hemianopia. Am J Ophthalmol 54:1091, 1962 117. Kerr FWL: A mechanism to account for frontal headache in cases of posterior fossa
tumors. J Neurosurg 18:605, 1961 118. Edmeads J: Headaches and head pains associated with diseases of the cervical spine. Med Clin North Am 62:533, 1978 119. Rosenberg M, Hoyt CS, King JS, et al: Treatment of chronic ocular pain by selective thermocoagulation of the
trigeminal ganglion. Am J Ophthalmol 91:526, 1981 |