




|
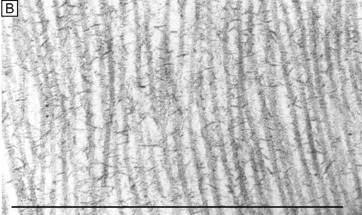
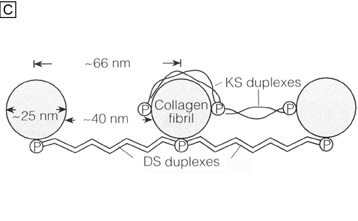
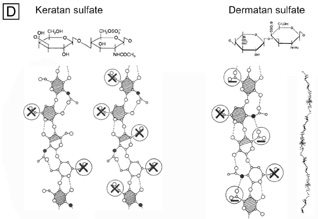
| Fig. 14. Frontal section TEMs (90,000×) of longitudinally running corneal stromal collagen fibrils in a lamellae without (A) and with (B) Cupromeronic blue staining. Notice the scattered “amorphous ground substance” in the interfibrillar spaces in (A), whereas in (B) duplexes of proteoglycans are clearly seen bridging collagen fibrils. Bars = 1 μm. (C) Diagram of how proteoglycans attach along the periphery of collagen fibrils via their core proteins (P) and how the GAGs duplex in an anti-parallel fashion in the interfibrillar space. (D) Diagram of the polymer backbones of keratan sulfate and dermatan sulfate. The top portion shows the primary structure of the repeating disaccharide units of keratan sulfate (top left) and dermatan sulfate (top right). The bottom portion of the diagram shows the secondary structures of each proteoglycan. Notice that in the human cornea ∼50% of keratan sulfate is in the normal-sulfated state (bottom, farthest left), whereas the other ∼50% is in the oversulfated state (bottom, center left). Also, notice that all three displayed proteoglycan polymers have similar backbones and therefore form similar secondary structures of a twofold helix (bottom, farthest right). Anionic charges = sulfate esters (X) and carboxylic acid (−). Bars = 1 μm. (C and D are modified from Scott JE. Proteoglycan: collagen interactions and corneal ultrastructure. Biochem Soc Trans 19:877, 1991.) |