LACRIMAL GLAND AND ACCESSORY GLANDS
The exocrine main lacrimal gland is located in the superotemporal orbit in a shallow lacrimal fossa of the frontal bone. It is surrounded by fibrous tissue that is attached superiorly to the frontal bone periosteum. In the adult, the lacrimal gland measures 20 mm by 12 mm by 5 mm and weighs approximately 0.78 g. It is incompletely divided by the lateral horn of the levator aponeurosis into a larger orbital lobe, which comprises 60% to 70% of its total mass, and a smaller palpebral lobe below.1,2 The two lobes are connected by vessels, nerves, and the lacrimal ducts. The orbital lobe lies posterior to the orbital septum and preaponeurotic fat, and anterior to the levator aponeurosis.1 With increasing age, the gland may herniate through a weakened orbital septum, resulting in fullness of the lateral upper eyelid. The palpebral lobe is located posterior to the levator aponeurosis in the subaponeurotic space and directly anterior to the conjunctiva, which is adherent to the posterior aspect of the gland. Medially, Müller's muscle may intervene between the posterior gland and the conjunctiva. Anteriorly, the palpebral lobe may extend beyond the orbital margin to lie in the superolateral fornix, where it may be visible through the conjunctiva with eyelid eversion.
The lacrimal gland is divided into numerous lobules, separated by interlobular fibrovascular connective tissue. Each lobule is structurally composed of the acinar unit and the ductal system. Two to six secretory ducts from the orbital lobe of the lacrimal gland pass through the palpebral lobe or along its fibrous capsule, joining with ducts from the palpebral lobe to form 6 to 12 tubules that empty into the superolateral conjunctival fornix 4 to 5 mm above the tarsus.3,4 Excision of the palpebral lobe may therefore interrupt drainage from the orbital lobe. The ducts, lined by pseudostratified, nonkeratinizing squamous epithelium, form a branching pattern within the lacrimal gland. Secretion from the acinar units first drains into the smaller intralobular ducts, proceeds through the larger interlobular ducts, and eventually passes into the main excretory ducts before draining into the superolateral conjunctival fornix. The surface of the ductal lumina contains microvilli, similar to the acinar secretory cells.5,6 The epithelial walls of the larger ducts contain three to four layers of columnar or cuboidal cells, compared with a single layer in the smaller intralobular ducts.
Each acinar unit, or secretory unit, of the lacrimal gland is composed of an inner layer of columnar or pyramidal-shaped secretory cells around a central lumen, and a surrounding basal layer of myoepithelial cells. The nucleus is located at the base of the acinar secretory cell, and numerous membrane-bound zymogenic secretory granules are present in the apical cytoplasm.7 The number and size of granules, which contain predominantly serous proteins for secretion, vary from cell to cell. Typically, larger granules are located in the peripheral lobules.6,8 The zymogenic secretory granules fuse with the apical cell membrane and release their granular contents into the acinar lumen through a mechanism known as emiocytosis.5,6,9 Myoepithelial cells are spindle- or basket-shaped cells, composed of cytoplasmic myofilaments, located between the base of the secretory cell and the basement membrane of the acinar unit.7 Contraction of the myoepithelial cells helps drive secretions into the drainage tubules and ducts.
Accessory lacrimal glands are located in the conjunctival fornices and along the superior tarsal border. There are approximately 20 to 40 accessory glands of Krause in the superior conjunctival fornix. There are approximately half the number of glands of Krause present in the lower eyelid as in the superior conjunctiva. Accessory glands of Wolfring, which are slightly larger, are located along the superior tarsal border in the upper eyelid, with 4 to 20 located in the upper eyelid, and 2 to 4 in the lower eyelid.1,3,10,11 The accessory lacrimal gland lobules empty onto the conjunctiva surface through small branching ducts that drain into a single excretory duct that is lined by one or two layers of cells.11
Like the main lacrimal gland, the accessory lacrimal glands are also innervated. Parasympathetic and sympathetic nerves have been identified in accessory lacrimal glands of humans.11–14 The presence of nerves suggests that secretion from accessory lacrimal glands is neurally regulated. Therefore, the accessory lacrimal glands can also contribute to stimulated tear secretion.
INNERVATION OF THE SECRETORY SYSTEM
The lacrimal gland receives innervation from cranial nerves V and VII, as well as from the sympathetics of the superior cervical ganglion.15 The lacrimal branch of the ophthalmic division of the trigeminal nerve carries sensory stimuli from the lacrimal gland, with the cell bodies located within the Gasserian ganglion. Afferent sensory fibers exit the posterior aspect of the lacrimal gland, travel along the superior border of the lateral rectus muscle, and exit the orbit through the superior orbital fissure.
Parasympathetic secretomotor fibers originate in the lacrimal nucleus of the caudal pons and exit the pontomedullary junction between cranial nerves VI and VIII. The fibers enter the internal auditory canal and travel a long course within the nervus intermedius, the greater superficial petrosal nerve, the deep petrosal nerve, and the vidian nerve to finally synapse in the pterygopalatine ganglion.4 The greater superficial petrosal nerve arises from the preganglionic parasympathetic fibers of the geniculate ganglion. The deep petrosal nerve carries sympathetic fibers from the internal carotid plexus. Postganglionic parasympathetic fibers leave the pterygopalatine ganglion through the pterygopalatine nerves to innervate the lacrimal gland.16,17 Some fibers may join the zygomatic nerve as it branches from the maxillary division of the trigeminal nerve and enters the orbit through the inferior orbital fissure. Branches of the zygomatic nerve may ascend and enter the posterior surface of the lacrimal gland either alone or in combination with the lacrimal nerve.1 Traditionally, it was thought that secretomotor nerves pass to the gland through the zygomatic and lacrimal nerves. More recently, Ruskell18 found that parasympathetic fibers traveled along a branch of the middle meningeal artery through the superior orbital fissure before joining the ophthalmic or lacrimal artery to supply the lacrimal gland. After parasympathetic denervation, the lacrimal gland has been reported to be supersensitive to parasympathomimetic agents such as topical or systemic pilocarpine.19,20
Postganglionic sympathetic nerves, arising from the superior cervical ganglion and internal carotid artery plexus, arrive with the lacrimal artery branch of the ophthalmic artery. Sympathetic nerve fibers also arrive along with parasympathetics in the zygomatic nerve. The zygomatic branch of the maxillary trigeminal nerve gives off the lacrimal branch before dividing into zygomaticotemporal and zygomaticofacial branches. This lacrimal branch anastomoses with the lacrimal nerve of the ophthalmic trigeminal nerve or travels along the periorbita to independently enter the gland at its posterolateral aspect.
The lacrimal gland receives arterial supply from the lacrimal artery of the ophthalmic artery, with contributions from the recurrent meningeal artery and a branch of the infraorbital artery. The arterial supply enters the posterior aspect of the gland and eventually continues anteriorly as the arterial arcades of the eyelids. The venous drainage follows approximately the same intraorbital course of the artery and drains into the superior ophthalmic vein and cavernous sinus. Lymphatic channels in the interstitial tissue between the gland lobules join the lymphatic drainage from the lateral eyelids and conjunctiva to empty into the preauricular lymph nodes.
REGULATION OF SECRETION
The lacrimal gland secretes water, electrolytes, and protein in response to neural and hormonal stimulation and is the major contributor to the aqueous portion of the tear film with additional contributions from the accessory lacrimal glands, the cornea, and the conjunctiva.21 Thus, the lacrimal gland is extensively innervated with parasympathetic, sympathetic, and sensory nerves. Parasympathetic nerves, containing acetylcholine (ACh) and vasoactive intestinal peptide (VIP), and sympathetic nerves containing norepinephrine, are potent stimuli of lacrimal gland secretion.21 In addition, many hormones from the hypothalamic-pituitary-gonadal axis have significant influences on the lacrimal gland, including stimulating secretion of secretory immunoglobulin A (SIgA).22–24 Sensory nerves are the least prevalent and release substance P and calcitonin gene-related peptide. In the lacrimal gland, each of these agonists bind to specific receptors on the acinar cells and initiate a different cascade of intracellular events, or cellular signal transduction pathway. Stimulation of sensory nerves in the cornea or conjunctiva carries the afferent limb of the tear reflex arc, and the efferent parasympathetic and sympathetic nerves subsequently stimulate lacrimal gland secretion.
The lacrimal gland consists of three main types of cells: acinar, ductal, and myoepithelial cells. Plasma cells, lymphocytes, macrophages, and mast cells are also present. Myoepithelial cells are basket-shaped cells that surround the acinar cells. Because of the abundance of α-smooth muscle actin present in these cells, it is believed that they are involved in contraction to expel the secretory product. In support of this hypothesis, these cells have been shown to express several neurotransmitter receptors.25,26 Ductal epithelial cells form the lacrimal ducts and modify the secretory product as it moves through the ducts. The acinar cells are the predominant cell type in the lacrimal gland and make up approximately 80% of the cells in the gland. These cells form the secretory unit of the gland termed an acinus. In cross-section, acini are seen as a ring of pyramidal-shaped cells joined by tight junctions on the lateral side.21 The presence of tight junctions results in the formation of highly polarized cells and division of the plasma membrane into apical membranes (membranes bordering the ducts), lateral membranes (membranes between adjacent cells), and basal membranes (membranes that are exposed to extracellular matrix and blood supply). This formation ensures unidirectional secretion of the secretory product toward the ductal lumen. Receptors for hormones and neurotransmitters are located on the basolateral membranes. Once activated, the signal from these receptors is transduced through the cell leading to fusion of secretory vesicles with the apical membrane to release their contents into the ducts. Ductal cells modify the secretory product before it is released onto the ocular surface. Electrolyte concentration of the secreted lacrimal gland fluid varies with flow rate, with hypertonic fluid produced during low flow rates and isotonic fluid at faster flow rates.27 The secretion of electrolytes and water into the lumen is dependent on the activation of ion transport proteins and ion channels also located in the apical and basolateral membranes.
The lacrimal gland is able to secrete proteins by two different processes, each of which is regulated differently. In the first process, known as constitutive secretion, the proteins are synthesized in the endoplasmic reticulum, modified in the Golgi apparatus, and sorted into secretory vesicles. These vesicles immediately fuse with the apical membrane.22–24 These vesicles are not stored; thus, regulation of constitutive secretion occurs at the level of protein synthesis. An example of a constitutively secreted protein is SIgA. The second type of secretion is called regulated protein secretion. In this type of secretion, the proteins are synthesized in the endoplasmic reticulum, modified in the Golgi apparatus, and packaged into secretory vesicles. These vesicles are stored until the appropriate stimulus, such as the release of neurotransmitters from nerves, occurs, at which time they fuse with the apical membrane. The lacrimal gland secretes in a merocrine fashion, that is, only a small portion of granules fuse on stimulation.
Clinically, tear secretion from the main lacrimal gland may be stimulated by reflex tearing from stimulation of the conjunctiva and cornea by irritating phenomena, psychogenic tearing, or aberrant regeneration. Reflex tearing may also occur by stimulation of the retina and optic nerve by bright light, and by spicy foods because of a reflex arc between the gustatory and the lacrimal nucleus. In psychogenic tearing, strong emotions and stress cause afferent pathways from the frontal lobe, basal ganglia, thalamus, and hypothalamus to interact with the lacrimal nucleus.28 Regeneration of injured parasympathetic secretomotor fibers destined for the salivary glands may aberrantly innervate the lacrimal gland, resulting in crocodile tears, or frank epiphora with gustatory stimulation. These stimuli affect the reflex tear production from the main lacrimal gland, with the continuous basal tear secretion from the accessory lacrimal glands, conjunctival goblet cells, and sebaceous glands all left unaffected.
Excision of the main lacrimal gland may result in lacrimal insufficiency, with profound changes in water and electrolyte secretion, loss of the normal tear film, and corneal sequelae.29,30 However, total removal of the main lacrimal gland does not in itself lead to keratoconjunctivitis sicca, which suggests that tear production from the accessory lacrimal glands may be sufficient to maintain a stable tear layer on the cornea.31
STIMULATION OF PROTEIN SECRETION BY PARASYMPATHETIC NERVES
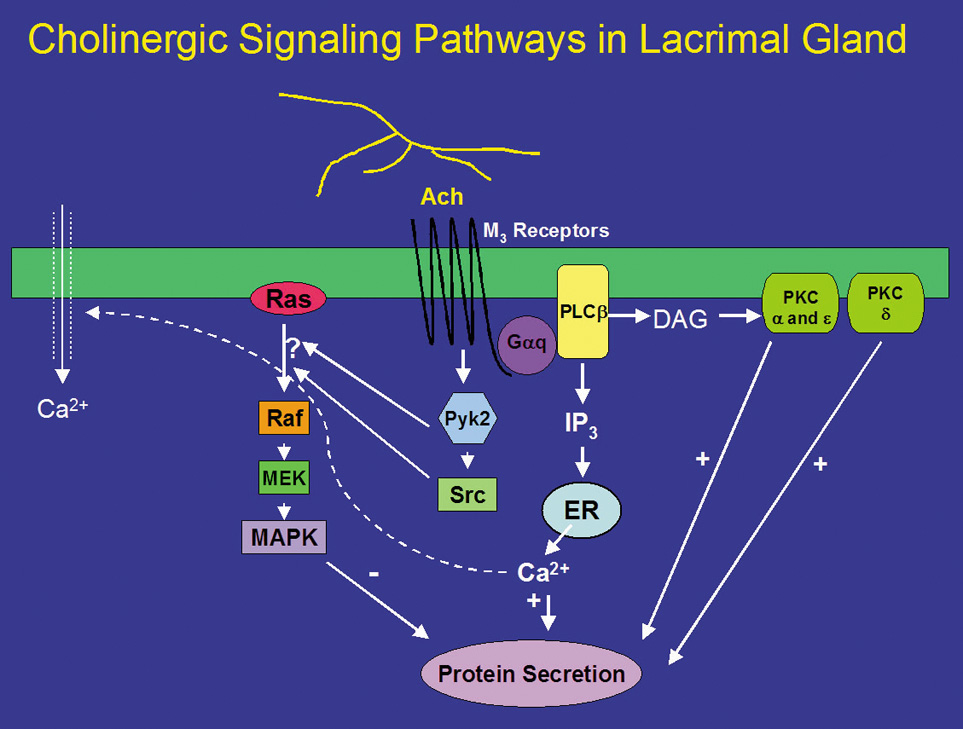
Neurotransmitters released from parasympathetic nerves are potent stimuli of lacrimal gland secretion. In the lacrimal gland, these nerves contain ACh and VIP that activate two distinct intracellular signaling pathways. ACh binds to M3 muscarinic receptors present on basal and lateral membranes of acinar cells (Fig. 1).32 The activated M3 muscarinic receptor is coupled to the G protein, Gαq/11.33–36 The G protein, in turns, activates phospholipase C (PLC). PLC hydrolyzes phosphatidylinositol bisphosphate (PIP2), a membrane phospholipid, into two second messengers, 1,4,5-inositol trisphosphate (1,4,5-IP3) and diacylglycerol (DAG).32,37–40 1,4,5-IP3 binds to IP3 receptors on the endoplasmic reticulum to release its Ca2+ stores, increasing the intracellular concentration of calcium, or [Ca2+]i.41 In the lacrimal gland, cholinergic agonists increase 1,4,5-IP3 within seconds resulting in a rapid increase in [Ca2+]i.41 When the intracellular Ca2+ stores are depleted, a feedback mechanism is activated whereby Ca2+ enters the cells through Ca2+ channels in the plasma membrane of the acinar cell.42,43 This Ca2+ influx is termed “stored operated Ca2+ entry” or “capacitative Ca2+ influx” and is responsible for the slower, sustained phase of increased [Ca2+]i. Therefore, the cholinergic agonist-stimulated Ca2+ response is a biphasic response of a rapid burst released from intracellular stores followed by movement of Ca2+ into the cell that is sustained until the stimulus is removed or decreases slowly over time.41,44 In conjunction with calmodulin, the Ca2+ phosphorylates and therefore activates proteins, as of yet unidentified, that lead to protein secretion (Fig. 1). The increase in intracellular calcium is necessary for both muscarinic and adrenergic agonist-evoked exocytosis from acinar cells, and may even be sufficient to induce exocytosis in the absence of agonist stimulation.45
DAG, which is also produced on hydrolysis of PIP2, binds to and activates several members of the protein kinase C (PKC) family.46 The PKC family includes 10 different isoforms and has been divided into three groups depending on cofactor requirements.47–50 Classic PKCs include PKCα, -βI, -βII, and -γ and require calcium and phospholipids for activation. Novel PKC isoforms include PKCδ, ε, η, and θ, and are calcium independent but phospholipid dependent. Atypical PKC isoforms are PKCλ and ζ. These isoforms are calcium and phospholipid independent. The lacrimal gland contains at least four PKC isoforms, PKCα, δ, ε, and λ. PKD, also known as PKCμ, is also present in the lacrimal gland.51 In general, PKC isoforms have cell-specific localizations and functions, and the lacrimal gland is no exception.51 In the lacrimal gland, PKCα was found to be located in the cytoplasm and plasma membranes of acinar cells. PKCδ was found in the cytoplasm of acinar and myoepithelial cells. PKCε was located in the cytoplasm, basal, and lateral membranes, and on an apical membrane system that is similar to actin networks of acinar cells. PKCε was also seen occasionally on myoepithelial cells. PKCλ was observed in acinar cells on an endomembrane system, potentially the endoplasmic reticulum or Golgi apparatus.51
Translocation of PKC from the cytosol to membrane fractions can be an indication of activation. Stimulation of lacrimal gland acini with cholinergic agonists for less than 1 minute translocates PKCα from the cytosol to the basal and lateral membranes.52 Cholinergic agonists also translocate PKCδ and ε, albeit with a longer time course.53 With specific inhibitors of the α, δ, and ε isoforms of PKC, cholinergic agonists were shown to use all three isoforms to varying degrees to stimulate secretion. Inhibition of PKCα reduced cholinergic agonist-induced protein secretion approximately 75%, whereas inhibition of PKCε reduced cholinergic agonist-induced secretion by approximately 50%. Inhibition of PKCδ reduced cholinergic agonist-induced secretion by approximately 20%.53 These findings were confirmed by down-regulating PKC using phorbol esters. Down-regulation with phorbol esters decreased the amount of PKCα by 79%, PKCδ by 53%, and PKCε by 10%.54 This treatment also decreased cholinergic agonist-stimulated protein secretion more than 90%.54 Thus PKCα, δ, and ε play differential, but overlapping, roles in cholinergic agonist-stimulated protein secretion (Fig. 1).51,55,56
In summary, cholinergic agonists bind to M3 muscarinic receptors to activate PLC. PLC hydrolyses PIP2 to produce 1,4,5-IP3 and DAG. 1,4,5-IP3 increases [Ca2+]i, whereas DAG activates PKCα, δ, and ε. Thus, activation of protein secretion stimulated by cholinergic agonists uses at least three PKC isoforms along with Ca2+ to stimulate protein secretion (Fig. 1).
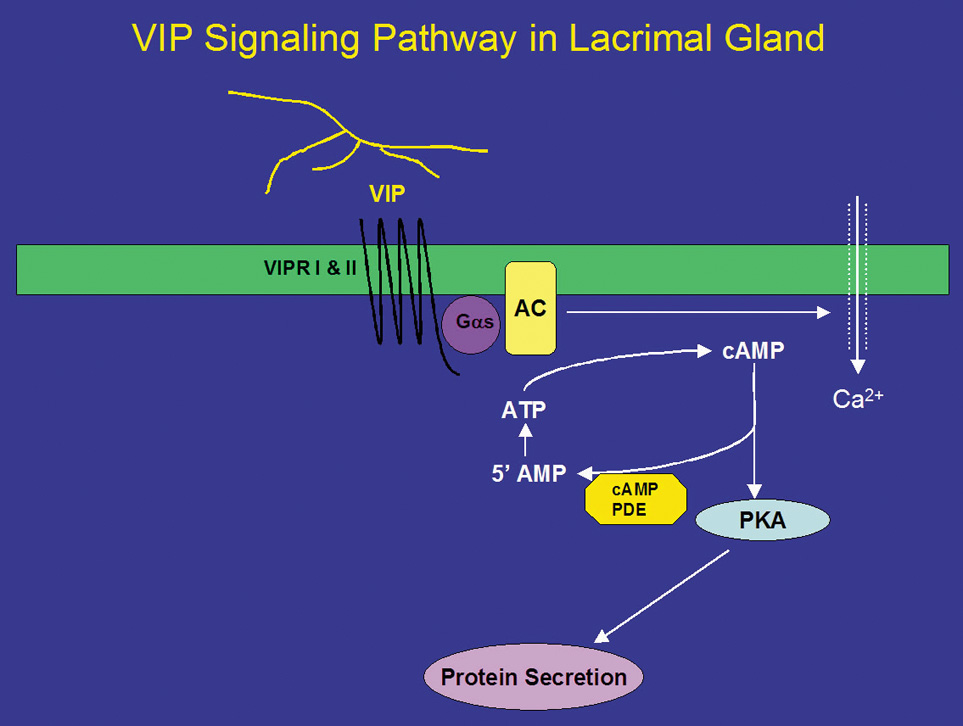
In addition to ACh, parasympathetic nerves also release the neuropeptide VIP on stimulation. VIP is a 28-amino acid regulatory peptide that is found in parasympathetic nerves of most exocrine glands. VIP binds to VIP receptors (VIPR) of which two types have been identified. These receptors are called VIPR1 and VIPR2.26 In the lacrimal gland, VIPR1 is present on the basolateral membranes of acinar cells, whereas VIPR2 is found on myoepithelial cells.26 VIPRs are coupled to the G protein, Gαs, to activate adenylyl cyclase (AC) (Fig. 2).26,35 The lacrimal gland contains at least three of the six known isoforms (I-VI) of AC, namely, ACII, ACIII, and ACIV. The presence of the other AC isoforms has not been detected in the lacrimal gland.26 Interestingly, none of the isoforms were localized to the plasma membrane as would be expected for interaction with Gαs. ACII was present almost exclusively on myoepithelial cells, whereas AC III was found only on the occasional duct, blood vessel, and myoepithelial cell. ACIV was found to be present in acinar cells but with an intracellular localization that appeared to be either endoplasmic reticulum or Golgi apparatus. It is possible that another AC isoform is responsible for the coupling of Gαs to its downstream effects or that ACIII is recruited to the plasma membrane on stimulation. This has been shown to be the case for muscarinic receptors in the lacrimal gland.57 Activation of AC leads to the formation of cyclic-adenosine monophosphate (cAMP) from AMP. cAMP, in turn, binds to and activates a cAMP-dependent protein kinase, known as protein kinase A (PKA) (Fig. 2). PKA is responsible for phosphorylating proteins that are components of the protein secretory machinery or ion transport channels necessary for secretion to occur, although none of the substrates have been identified in the lacrimal gland. Inhibition of PKA in the lacrimal gland with a specific inhibitor decreased VIP-stimulated protein secretion by 70%.26
The signal is terminated when cAMP is degraded by cAMP-dependent phosphodiesterases, which are present in the cell. Eleven different families of phosphodiesterases have been identified, each with varying specificities to cAMP and cyclic guanosine monophosphate.58 However, the types of phosphodiesterases present in the lacrimal gland remain unidentified. It is known that the nonspecific phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) stimulates secretion in the absence of agonists implying that the basal rate of secretion from the lacrimal gland is partially dependent on cyclic nucleotides. The effects of IBMX are also dependent on Ca2+ because removal of Ca2+ partially reduces IBMX-stimulated protein secretion.59
In addition to activation of the cAMP-dependent pathway, addition of VIP to lacrimal gland acinar cells also increases [Ca2+]i.41 The increase in [Ca2+]i obtained in the presence of VIP is 25% of the increase obtained when the cells were stimulated with cholinergic agonists.26,41 Unlike cholinergic agonists, VIP does not increase levels of IP3 but increases [Ca2+]i by modulating Ca2+ influx.60 Increased cAMP leads to increased intracytoplasmic concentrations of Ca2+ through plasma membrane channels and activates Ca2+-dependent K+ channels that play an essential role in fluid secretion.61
In addition to VIP, two other peptide hormones, α-melanocyte-stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH) stimulate lacrimal gland protein secretion.62 These peptides are not released from nerves, but are delivered by the blood supply to the lacrimal gland. α-MSH and ACTH stimulate protein secretion by activation of the cAMP-dependent pathway. Only the melanocortin 5 receptor, which can bind both α-MSH and ACTH, has been identified in the lacrimal gland.63,64 This receptor could mediate the effects of both hormones in the lacrimal gland.
STIMULATION OF PROTEIN SECRETION BY SYMPATHETIC NERVES
Norepinephrine, the neurotransmitter released from sympathetic nerves, stimulates lacrimal gland protein secretion.65,66 Norepinephrine can bind to both α- and β-adrenergic receptors present in the gland. Activation of β-adrenergic receptors causes an increase in protein secretion, which appears to occur through the cAMP-dependent pathway67 similar to the VIP pathway shown in Figure 2.
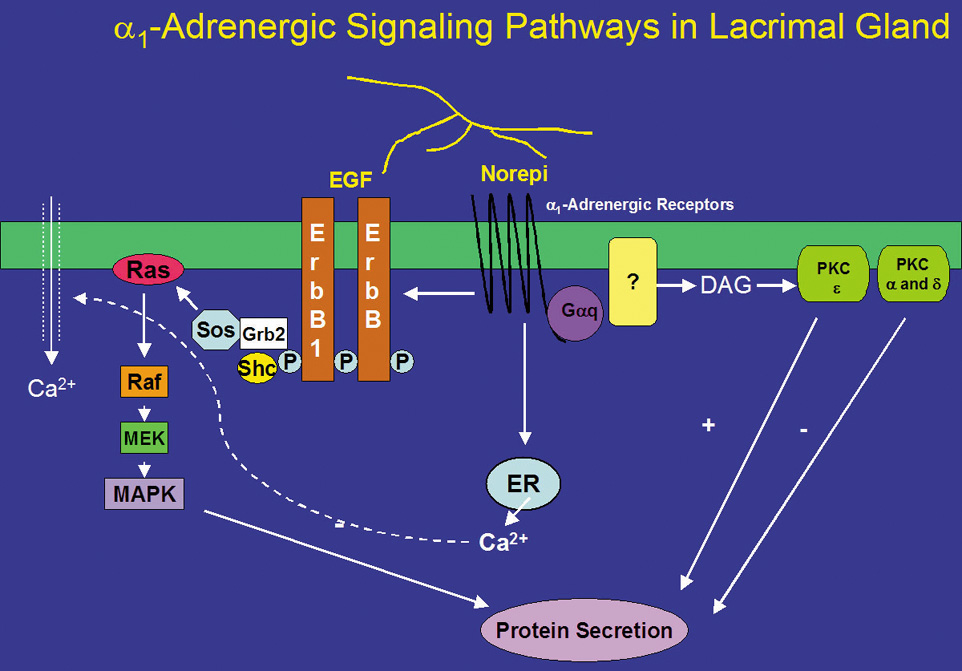
Norepinephrine, when bound to α1-adrenergic receptors in the lacrimal gland, is a potent stimulator of protein secretion. However, the signal transduction pathways used by α-adrenergic receptors are not completely understood. α1-Adrenergic receptors, similar to muscarinic receptors, activate the G-protein Gαq/11 (Fig 3).68 In contrast with muscarinic receptors, activation of Gαq/11 by α1-adrenergic receptors only accounts for a small portion (∼30%) of adrenergic agonist-induced secretion.68 The Gαq/11 presumably activates an unknown effector phospholipase, although it is known that the phospholipase is neither PLC nor phospholipase D (PLD).41,69 α1-Adrenergic agonists do not increase IP3 or IP4.50,70 However, they do cause a small increase in intracellular Ca2+, contributing only 20% of that caused by cholinergic agonists.70,71 The increased Ca2+ and activated PKC then result in protein secretion. In addition, α1-adrenergic agonist-induced protein secretion was not inhibited by chelation of extracellular Ca2+, indicating that the increase in [Ca2+]i does not play a major role in this secretion.70
Like cholinergic agonists, α1-adrenergic agonists have also been shown to influence protein secretion by PKC. Down-regulation of PKC with phorbol esters, however, increased α1-adrenergic agonist-induced protein secretion.72 By using inhibitors specific to PKCα, δ, and ε, Zoukhri et al showed that inhibition of PKCα and δ increased α1-adrenergic agonist-induced protein secretion, and that inhibition of PKCε decreased this secretion.53 These results suggest that α1-adrenergic agonists activate PKCα, δ, and ε, but only PKCε stimulates α1-adrenergic agonist-induced protein secretion whereas PKCα and δ inhibit it (Fig. 3). Thus, muscarinic and α1-adrenergic receptors used the same PKC isoforms but in different ways to control protein secretion. This suggests that cellular localization of PKC, through anchoring proteins, is vital for agonist-induced protein secretion.
STIMULATION OF PROTEIN SECRETION BY GROWTH FACTORS
Growth factors, such as the epidermal growth factor (EGF) family of growth factors, have been identified as stimuli of protein secretion from the lacrimal gland.73 This family includes EGF, transforming growth factor (TGF)-α, heparin-binding EGF (HB-EGF), heregulin, and other members of its growth factor family. These molecules are synthesized as transmembrane precursor molecules that are proteolytically cleaved to the soluble pro-form of the growth factor. For EGF, it is further cleaved to the mature form. The transmembrane precursor molecule and the pro- and mature forms are catalytically active and capable of binding to the EGF receptor subtypes.
There are four types of the EGF receptors, referred to as erbB1-4. These receptors are tyrosine kinases. On ligand binding, the erbB receptors form homo- and heterodimers with each other and phosphorylate one another on multiple tyrosine residues. Depending on the tyrosine residues phosphorylated, different adaptor proteins are recruited resulting in the activation of a physiologic process. The dimers can bind different ligands, recruit different adaptor molecules, activate different processes, and have varying potencies.74 The lacrimal gland contains all four types of erbB receptors, although their locations differ.73,75 ErbB1, also known as the EGF receptor, is present on the apical, basal, and lateral membranes of acinar and ductal cells, whereas erbB2 (known as HER2) is located on the basal and lateral membranes of acinar and ductal cells. The location of erbB3 has been reported to be on ductal cells,75 and erbB4 (known as Neu) is found on the apical, basal, and lateral membranes of ductal cells.73
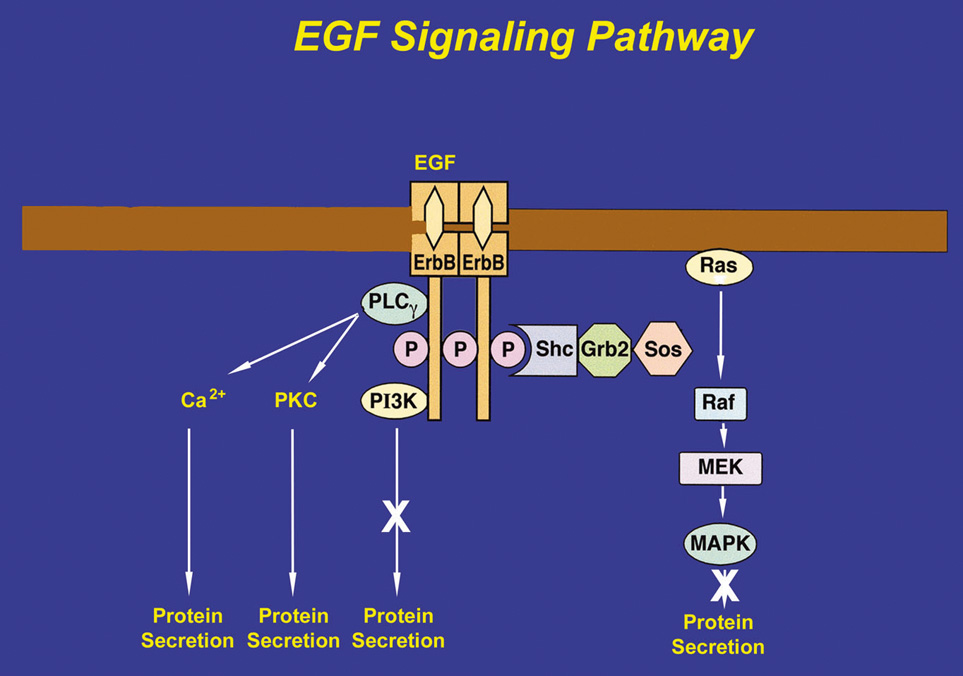
After EGF binds to an erbB receptor and forms a dimer with another erbB receptor, several adaptor proteins can be recruited, each linking the receptor to a different signaling pathway. To induce lacrimal gland protein secretion, EGF binds to the erbB1 receptor and recruits PLCγ, which, in turn, produces 1,4,5-IP3 and DAG. 1,4,5-IP3 increases [Ca2+]i, whereas the DAG activates PKC (Fig. 4).54 EGF therefore stimulates protein secretion through Ca2+ and PKC signaling pathways. Studies using chelation of Ca2+ and down-regulation of PKC were found to inhibit EGF-stimulated protein secretion.54 Heregulin, HB-EGF, and TGFα also stimulate protein secretion, although the precise cellular mechanisms have not been determined.73
If the adaptor proteins, Shc and Grb2, are recruited and tyrosine-phosphorylated by the erbB receptor, the protein SOS binds to Grb2. SOS catalyzes the exchange of GDP for GTP on Ras to activate it. Ras initiates the activation of a series of serine-threonine kinases including Raf (a mitogen-activated protein kinase [MAPK]), which in turn activates MEK (another MAPK), ultimately leading to activation of p42/p44 MAPK. Activated p42/p44 MAPK can then translocate to the nucleus to influence gene expression to initiate cell proliferation, migration, and apoptosis. Activated p42/p44 MAPK may also remain in the cytosol to influence secretion and other short-term processes.76–78 In the lacrimal gland, EGF causes the tyrosine-phosphorylation of Shc and Grb2, indicating that this pathway is activated.78 However, EGF activation of p42/p44 MAPK does not cause protein secretion, although it does modify cholinergic- and α1-adrenergic agonist-induced protein secretion. Heregulin, HB-EGF, and TGFα also activate p42/p44 MAPK in the lacrimal gland.73
The third protein that can be recruited to the erbB receptors is phosphatidylinositol 3-kinase (PI-3K). PI-3K phosphorylates several lipids including phosphatidylinositol 4,5-bisphosphate to produce phosphatidylinositol 3,4,5-trisphosphate. This stimulates processes such as cell growth, survival, and adhesion. Inhibitors of PI-3K do not have any effect on EGF-stimulated protein secretion in the lacrimal gland, which implies that either PI-3K is not recruited by erbB receptors in the lacrimal gland or PI-3K is recruited for another function other than secretion.54
In many different types of tissues, including the lacrimal gland, the signal transduction pathways of EGF and G-protein coupled receptors interact with one another. Indeed, in the lacrimal gland, inhibition of MEK increased cholinergic and α1-adrenergic agonist-induced protein secretion suggesting that activation of p42/p44 MAPK negatively modulates agonist-stimulated secretion.78 Cholinergic agonists have been shown to activate p42/p44 MAPK through activation of the nonreceptor tyrosine kinases, Pyk2 and Src (Fig. 1).78 In contrast, α1-adrenergic agonists use a different mechanism to activate p42/p44 MAPK. These agonists activate EGF to recruit Shc and Grb2, which activate the cascade of kinases that result in activation of p42/p44 MAPK (Fig. 2).78
Neuropeptides, such as substance P and calcitonin gene-related peptide, may indirectly affect lacrimal gland secretion. Substance P may stimulate protein secretion indirectly by causing mast cell degranulation, with the released mediator, serotonin, stimulating the lacrimal acinar cells. In contrast, calcitonin gene-related peptide inhibits degranulation of mast cells, resulting in decreased serotonin release and thus decreased protein release from the acini.79
REGULATION OF CONSTITUTIVE PROTEIN SECRETION
Proteins secreted through the constitutive pathway are secreted shortly after synthesis rather than being stored in secretory vesicles. The regulation of this secretion lies at the level of gene transcription and translation. SIgA and secretory component (SC) are examples of proteins secreted by this pathway. SIgA is composed of IgA and a J chain coupled to SC. SC is synthesized in the acinar and ductal cells, whereas IgA and the J chain are synthesized by plasma cells present in the lacrimal gland.22 SC is synthesized as a precursor molecule, known as the polymeric immunoglobulin receptor, which inserts into the basal and lateral membranes. IgA binds to this receptor and is endocytosed into distinct vesicles that are transported directly to the apical membrane. These vesicles fuse with the apical membrane releasing SIgA into the lumen.
Androgens play a significant role in the maintenance and function of the lacrimal gland.23 Dihydrotestosterone has been shown to increase both the synthesis and secretion of SC.80 Androgens appear to regulate this pathway by diffusing to the nucleus of the acinar cell where they bind to specific receptors.23 The receptors are members of a family of ligand-activated transcription factors, and when activated by androgens from the bloodstream, they regulate SIgA by controlling the rate of synthesis of the SC.23
SIgA antibodies act to inhibit viral, bacterial, and parasitic invasion, and damage to the lacrimal gland and ocular surface. Fluid secretion of the lacrimal gland is dependent on the presence of androgens; ovariectomy of female rabbits has been demonstrated to cause decreased tear secretion from the lacrimal gland.81 Likewise, the predominance of dry eyes in postmenopausal or pregnant women, or those taking oral contraceptives, suggests that the decrease in circulating androgens because of decreased function of the ovaries affects lacrimal tear production to some degree.82 Circulating androgens also exert an anti-inflammatory effect on the lacrimal gland by androgen-dependent production of anti-inflammatory cytokines (e.g., TGFβ) that suppress lymphocytic inflammation.23,82
MECHANISM OF WATER AND ELECTROLYTE SECRETION
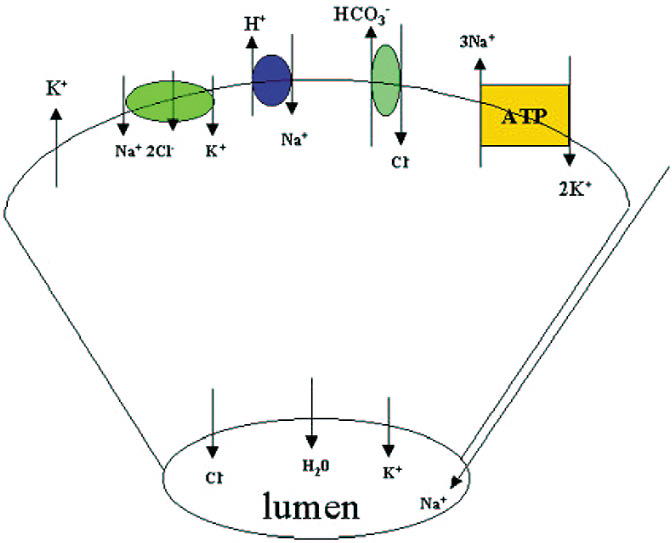
Along with protein, the lacrimal gland also secretes water and electrolytes. Acinar cells secrete fluid with an electrolyte composition similar to plasma. The increase in [Ca2+]i produced by cholinergic and α1-adrenergic agonists, as well as the increase in cAMP induced by VIP, activates K+ channels in the basal and lateral membranes and Cl− channels in the apical membranes of the acinar cells (Fig. 5).83 The result is a net efflux of K+ and Cl− into the lumen. Movement of these ions creates a negative potential difference causing Na+ to move into the lumen by a paracellular pathway through channels located between the cells.84 Water is then secreted into the lumen through water channels called aquaporins.85 The Na+/K+/Cl− cotransporter, Cl−/HCO3− exchangers, and Na+/H+ exchangers replace the Cl− that exited the cell through the apical membrane. The increase in Na+ as a result of the Na+/K+/Cl− cotransporter and Na+/H+ activates the Na+-K+-ATPase in acinar cell membranes. This latter transporter pumps Na+ out of the cell and K+ into the cell. Cl− enters the cell as its transport is coupled to Na+ movement. Cl−/HCO3− and Na+/H+ exchangers extrude HCO3− from the cell and are replaced by cellular carbonic anhydrase. As the fluid passes through the ducts, the ductal epithelial cells modify the fluid by secreting a fluid rich in KCl. The final fluid that exits onto the ocular surface is an isotonic solution containing Na+, K+, Cl−, and HCO3− (Fig. 5).86