IMAGES CAUSED BY THE RETINAL CIRCULATION One of the most easily demonstrated of the entoptic visualizations is the
subjective perception of the retinal blood vessels. Under ordinary
circumstances, the shadows cast by the retinal vessels remain in fixed
position relative to photoreceptors, even during eye movements. Because
the underlying neural network is adapted, the vessels are not subjectively
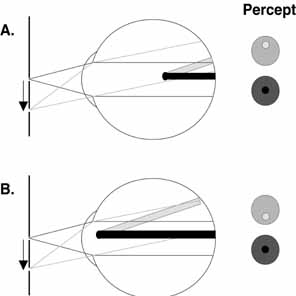
apparent in this situation. However, Purkinje noted that when
light enters the eye from an unusual angle (e.g., obliquely through
cornea or through the sclera) the shadows of the retinal vessels
fall on unadapted retina and the shadows of the retinal vessels become
visible for a brief period. This can occur, for example, during slit
lamp biomicroscopy of the anterior segment. The shadows of the arterial
and venous tree will be perceived as a greatly magnified, inverted
image that has come to be known as either Purkinje's tree or the
Purkinje figure. In this image the retinal vessels are observed arising
from the optic nerve head and branching out with finer and finer branches
extending toward the fovea. With this type of viewing, the foveal
pit, which lacks blood vessels, is readily distinguishable in this
image as a vacant area surrounded by the branching pattern. Helmholtz
made use of the parallax intrinsic to this phenomenon to demonstrate
that the light-sensitive elements in the retina lay within the
photoreceptor layer. To visualize the retinal vessels, place a small, bright light source over
closed eyelids near the limbal margins and oscillate the light source
at a relatively low frequency (2 to 4 Hz). In the resulting
entoptic image, the retinal vessels will appear as dark shadows against
a yellow–orange background. To be able to perceive greater
details in the image (such as the retinal capillaries), gaze
at a uniformly illuminated, bright surface through a pinhole positioned
at the anterior focal plane of the eye. The parallel light rays obtained
with this technique enhance the contrast of the image so that shadows
of vessels, including the capillaries surrounding the fovea, become
visible. With sustained viewing, the Purkinje figure will fade, but
the image can be regenerated by simply repositioning the illuminating
source. By rotating the light source in a circular pattern, image stabilization
can be avoided, and the shadows of the vessels will be perceived
continuously. Visualization of the retinal capillaries surrounding the fovea permits
estimation of the size and shape of the foveal avascular zone. Applegate
and colleagues6,7 used entoptic visualization of the retinal capillary net to measure the
size and shape of the foveal avascular zone. They determined that the
foveal avascular zone is an elliptical region with a mean diameter of
approximately 0.755 mm and a major axis approximately 17% longer
than the minor axis.7 These dimensions are consistent with foveal avascular zone diameters measured
by angiographic techniques.8,9 Zeffren and co-workers10 also found that the point of fixation often is not centered in the foveal
avascular zone. Furthermore, they observed that in a number of cases
the point of fixation is located eccentric enough within the region
that macular photocoagulation to within 200 μm of the center of the
region could result in a lesion at the point of fixation, thereby compromising
visual acuity. With conscious effort, pulsation of retinal blood vessels can be seen after
physical exertion or pressure on the globe. On close observation, it
is evident that: (1) the pulsation proceeds recurrently
along certain pathways; (2) the shadows are not seen in an area
close to the point of fixation; and (3) the movement is
not uniform but rhythmic or pulsatile. Two quick changes in the appearance
of the vascular network surrounding the fovea can become evident. In
one phase there is a rapid expansion of arterial tree that is synchronous
with cardiac systole and corresponds to the steep and rapidly
moving ascending limb of the pulse. This phase is immediately followed
by a slower contraction that corresponds to the descending limb of the
pulse wave. Because the period of the contraction is of longer duration
than the period of the expansion, the contraction often appears more
prominent. If accommodation is relaxed and a brightly illuminated uniform blue–violet (350 to 450 nm) surface (e.g., blue sky or blue
monitor) is viewed, then the circulation within the retinal capillaries
also can be visualized entoptically. Darting points or spots
that appear as bright circles with dark centers against a relatively
darker background will be visible. The darting points may appear to have
short tails and to follow sinusoidal paths similar in shape to the
retinal capillary loops evident on trypsin digest preparation. By viewing
the spots with one eye and the Purkinje tree with the other eye, Marshall11 found the spots to be on one plane and the superficial retinal vessels
on another plane. Therefore, he concluded that the spots were deeper
than superficial retinal vessels, possibly representing cells within the
deep capillary bed at the level of the inner plexiform and inner nuclear
layers. Considerable additional evidence has been accumulated to
suggest that this entoptic visualization results from the movement of
blood cells. This includes evidence that these darting points cannot
be seen in the central fovea and that pressure on the eye slows their
movement. In fact, with pressures in excess of 50 mm Hg, the movement
is halted. Although the type of blood cells responsible for this phenomenon
is still in question, evidence strongly favors leukocytes, because
the points are too infrequent to be erythrocytes. This could be explained
by assuming that erythrocytes are only visible when passing through
deep capillary bed. Hemoglobin, however, has an absorption peak in
the blue–violet range, so erythrocytes would cast relatively intense, continuous
shadows against a light background. Under the same
conditions, leukocytes would transmit blue–violet light and would
be seen, therefore, as bright interruptions in a continuous shadow. This
latter description is consistent with the subjective report of the
appearance of this entoptic image. Riva and Petrig12 developed a technique that uses the properties of this entoptic image
to estimate leukocyte velocity. Patients are asked to match the velocity
of global motion in a simulated pattern of random dots to the global
motion of the darting points in the entoptic image from their own eye. The
density of points (shadows) in the simulation, the time
average of the velocity waveform, and the pulsatility of the motion
are adjusted to match the patient's own entoptic visualization. Shadow
velocity is then used to estimate volume blood flow. The effects
of hyperoxia and hypoxia, changes in perfusion pressure, cigarette smoking, and
diseases affecting the visual system (e.g., diabetes
and glaucoma) on retinal blood flow have been studied using this
technique.13 With further intense concentration, the perception of these luminous darting
points may be observed to be followed by the visualization of a
darkened field that has the appearance of a turbulent surface of boiling
water with an abundance of smaller bright points moving much faster
than the points detected in the earlier image. Distinct islands of boiling
can be appreciated within the turbulent surface. Marshall11 describes this image as “A surging circulation in irregular sinuses
somewhat fanlike in appearance of a dark reddish-gray color
bounded by a black background meshwork. Over the field may also be seen
innumerable fine granules of black…but not always. The effect
lasts only a few seconds, but it may usually be repeated several times.” One
possible explanation of the source of this perception is
that it represents a visualization of circulation in the choriocapillaris. PHOSPHENES The term phosphenes (Greek for “to show light”) refers to the visual
sensations of light produced by nonphotic or nonluminant stimuli. Mechanical
and electrical energy as well as electromagnetic energy outside
the visible spectrum may be the source of different phosphenes. These
forms of energy often are termed “inadequate” stimuli because, under
most circumstances, they do not produce visual perception. Various forms of mechanical energy produce phosphenes. Pressing or rubbing
the eye, blunt trauma, strenuous exercise, sudden eye movements, and
prolonged accommodation or convergence all are known to bring about
phosphenes. Gentle, but forceful pressure to the nasal or temporal portion
of the eye elicits an immediate, very bright, well-circumscribed
ring of blue–white in the periphery of the opposite visual
field (Purkinje's blue ring). This is best seen in a
dark-adapted eye and has been attributed to a mechanical distortion
of the sensory elements in the retina, which then initiates a neural
response. When pressure on the globe is maintained for a prolonged
period (approximately 3 minutes) at a level that is sufficient
to create slight discomfort, a perception of a broad circular blue
ring often can be detected. This percept has been described as a broad
automobile tire somewhat flattened vertically. On careful observation, this
image may initially be observed to consist of two blue spots (one
nasal and one temporal) that expand slowly to take the
form of a broad arc. As the figure continues to grow, the arcs coalesce
into the blue circle by a slow motion (similar to water seeping
between two plates of glass). The center of the oval, which is devoid
of color, extends from approximately 2 degrees above and below the
fovea to approximately 3 degrees on either side. The periphery of the
oval is sharply outlined and extends to approximately 10 degrees vertically
and 12 degrees horizontally. As a result, this image approximates
the outer limits of the macula, with the blue circle covering the
retinal region of greatest rod density. Several different phosphenes are associated with eye movements. If an eye
movement is made while staring at an evenly illuminated bright surface, a
dark gray or pale blue oval area ringed by a bright blue–white
border may be visualized in the region of the blind spot of each
eye (the fiery rings of Purkinje). The rings appear accentuated
in the dark and often are more evident in the eye moving nasally. Generally, the
rings are perceived as larger than the blind spot.1 One possible cause of this phosphene is pressure on the retina adjacent
to the optic disk caused by traction from the optic nerve. During nasal
rotation, traction is exerted on the retina temporally adjacent to
the disk while the adjacent area on the nasal side is under compression. More
extensive eye movements produce similar phosphenes that are observed
farther in the periphery and may result from traction on the retina
by the extraocular muscles at their points of insertion adjacent
to the ora serrata. When dark-adapted, a different phosphene (Nebel's flick
phosphene) may be observed after a rapid flick or microsaccade.14 This phosphene typically is evident in people older than age 40 years
and is perceived as a brief (0.33 seconds), bright, blue or
orange, sheaf-like pattern extending 20 degrees to 40 degrees horizontally
and vertically and centered on a dark background. With repeated
observation, the color fades from this phosphene and the pattern
may shrink. The apex of the pattern points in the direction of the eye
movement and, similar to Purkinje's fiery rings, localizes at the
blind spot. Typically, this pattern is observed in both eyes, although
the pattern may be larger and brighter in the eye moving nasally. Nebel14 proposed that this phosphene is caused by a transient deformation of the
posterior vitreous face, perhaps because of early posterior vitreous
degeneration. In an early stage of posterior vitreous degeneration, there
may be a slight looseness and shrinkage of the posterior vitreous, decreasing
the normal slack and lag while increasing the force to which
the retina and vitreous attachments are subjected. Abrupt eye movements
transmit the inertial drag of the vitreous body to the retina at
the posterior pole (centered on the optic disk), causing deformation
of retinal structures similar to a mechanical phosphene. Degeneration of the vitreous with frank posterior detachment appears to
underlie the entoptic visualization known as Moore's lightning streaks. Mechanically
related to, but distinct from, Nebel's flick
phosphene, Moore's lightning streaks appear as flashes of light
that often are described as looking like lightning. Moore described them
as having a vertical direction and only occurring on the temporal side
of the eye.15 Verhoeff,16 however, found that they could occur in both the nasal and the temporal
visual fields. Verhoeff theorized that this entoptic phenomenon resulted
from degenerative condensation and vitreous shrinkage with separation
from the retina. The lightning streaks are generated when an eye
movement causes a sudden impact of condensed vitreous on the retinal periphery. Vitreous
traction on the retina was excluded by the fact that
the streaks appear on the side to which the ocular rotation is directed. Moore's
lightning streaks occur in association with the development
of opacities in the vitreous, seldom occur before middle age, and
are more frequent in females. Moore suggested that although the streaks
may persist for years (possibly becoming less frequent and
less brilliant with age), they are not precursors of retinal detachment
and they do not imply or herald any serous disease of the eye. In
an extended follow-up (18 years or more) of patients
reporting Moore's lightning streaks, Verhoeff16 was unable to find any patients returning with retinal detachments in
the affected eyes. In addition, Linder17 found that 35% of a series of patients (n = 115) with
posterior vitreous separation observed the lightning streaks; however, none
of these had a retinal detachment. These reports support
the belief that lightning streaks are innocuous. Nevertheless, individuals
reporting light flashes should receive careful and periodic examination
of the retina and vitreous to rule out the existence of significant
peripheral retinal degeneration or retinal detachment. In this regard, it
is important to differentiate the lightning streaks from nonspecific
flashes, which may indicate the presence of vitreoretinal pathology. Berens18 found that 7 of 36 patients reporting nonspecific light flashes had posterior
vitreous separation and rhegmatogenous retinal detachment, whereas
Morse and co-workers19 found that 23 of 100 patients reporting nonspecific light flashes had
vitreoretinal disease (16 with retinal breaks or holes). A sudden accommodative response also can produce a phosphene.20 This entoptic image appears similar to the image produced by a more forceful
eye rotation, but in this case the cause is most likely to be traction
of the ciliary muscle on the peripheral retina. This explanation
is consistent with evidence that the ora serrata moves forward during
accommodation (0.5 mm per diopter of accommodation). During
overaccommodation when viewing an object close to the limit of accommodation, the
whole field tends to darken somewhat nonuniformly. The
periphery is affected more, but a central patch is also dark. On further
accommodation, the central patch lightens in its center. No loss of
acuity is evident in the darkened areas. This phosphene can be appreciated
through an artificial pupil and the patchiness is not consistent
with the more general reduction in luminance associated with pupillary
constriction.
Electrical stimulation of the eye also produces phosphenes.21–22
When the terminals of a low-voltage cell (less than 10 volts) are placed
between the tongue and the upper lip while in the dark, a faint glow can
be observed all over the visual field. These phosphenes have been described
as being very distinctive and conspicuous, appearing almost as sharply
delineated as real objects. The phosphenes also are attractive, similar
to contour lines on topographic maps except that the lines do not cross
one another and they disappear either by moving off into the periphery
or by forming a loop that eventually contracts to nothing. With alternating
current, seven types of pattern may be observed. Four of the patterns
are noted when the eye is uniformly illuminated, whereas the other three
are detected in the dark-adapted eye. These patterns also appear to be
dependent on the frequency and the current of AC stimulation. Wolff and
colleagues21 suggested that the patterns
arise in the retina as a result of the alternating current acting on radially
oriented structures such as the photoreceptors and bipolar cells. Carpenter21–22
further suggested that different types of patterns arise within neuronal
domains responding to opposite phases of the current.
Exposing the eye to electromagnetic sources such as x-rays, cosmic
rays, and fast particles that are outside the visible spectrum also
causes phosphenes. The phosphene associated with exposure of the eye
to x-rays (100 to 0.01 nm) appears as a homogeneous luminous
blue–green or yellow–green glow that fills the entire
visual field and resembles an atmospheric electrical discharge behind
clouds on the horizon. The phosphene does not appear to be caused
by fluorescence of the retina or ocular media. Rather, it is thought that
x-rays have a direct ionizing effect on the rod and cone photopigments. Because
this phosphene is only perceived in eyes with light
perception, it has been used to test retinal function in eyes with
opaque media. In contrast to the phosphene elicited by exposure to x-rays, the
phosphene associated with radium exposure is a diffuse
homogeneous glow that is more green than blue. The radium phosphene is
primarily the result of beta particles acting on the ocular media to
induce fluorescence, although a lesser portion of the effect may be caused
by gamma rays acting in a fashion similar to x-rays. During space flight, astronauts have reported entoptic visualizations, many
of which only occur in the dark-adapted eye. These entoptic
phenomena have been described as colorless or blue-white
light flashes and streaks of light. The mechanism mediating these effects
is uncertain. However, based on evidence that cosmic rays can be
detected by the human eye,24 it has been speculated that cosmic particles that penetrate the space
capsule play a role in these visualizations. An alternative explanation
is that these phosphenes are caused by radiation that is produced whenever
a charged particle passes through a transparent medium (e.g., vitreous
or retina) at a speed exceeding the speed of light in
the same medium (Cerenkov radiation). This produces shock-wave
phenomena (the optical equivalent of the sonic boom) or
direct ionization with excitation of retinal molecules secondary
to high-energy proton recoils together with the release of alpha
particles by neutron reactions on carbon, nitrogen, and oxygen molecules. This
alternative is supported by evidence that neutrons, protons, alpha
particles, muons, and the nuclei of carbon, nitrogen, and hydrogen
cause similar sensations in ground-based simulations. The
visible sensations associated with Cerenkov radiation are only apparent
in the dark-adapted eye and include large crescent-shaped
and cloud-like flashes, brighter and smaller flashes, and
wide streaks or bands with dark centers. The visible sensations associated
with direct ionization and excitation include pinpoint and star-like
flashes and short light streaks. BLUE ARCS OF THE RETINA (PURKINJE)
During monocular viewing of a dim light of any color in a darkened room
with the temporal parafoveal retina stimulated (i.e., 1 degree to 2 degrees
from the fixation point), two faint glowing blue–gray arcs will
be seen bowing above and below fixation (Fig.
3). These arcs begin at the source (although they are noticeably wider
than the source) and extend to the blind spot. If the nasal parafovea is stimulated instead, then a triangular
patch of blue haze (a blue spike) with its base at the light source and
its apex at the blind spot will be observed. This entoptic phenomenon,
which is referred to as the blue arcs of the retina, appears briefly (0.5
to 1 second) but fades on extended observation. The position and orientation
of the blue arcs are generally held to correspond to the route of the
parafoveal arcuate nerve fiber bundles extending to the optic disk. Thus,
this visualization is thought to be the result of secondary electrical
stimulation of the retina whereby action potentials in the arcuate bundles
excite adjacent neurons. Moreland25,26
suggested that both rod and cone pathways are involved in generating this
entoptic image.
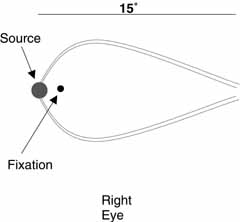 Fig. 3 An illustration of the appearance of the blue arcs of the retina when the
temporal parafoveal retina is stimulated. Fig. 3 An illustration of the appearance of the blue arcs of the retina when the
temporal parafoveal retina is stimulated.
|
POLARIZED LIGHT The eye's response to polarized light is evident in the entoptic phenomena
known as Haidinger's brushes. These entoptic images appear
as faint yellow and blue brush-like patterns in the central
visual field when a source of polarized light is viewed (Fig. 4). The images extend from the fixation point in a pattern similar
to a Maltese cross or a windmill, with the yellow brushes standing out
against a blue background when white light is used. Against a blue background, the
blue brushes may look black. The blue arms coincide with
the electric vector of the polarized light, whereas the yellow brushes
are oriented perpendicular to the polarity of the source. Although this
entoptic phenomenon may be seen in the naturally polarized light of
the sky it is more readily demonstrated by looking at a bright, diffuse
white or blue field through a sheet of polarizing material that is
continuously rotating. The rotation helps to prevent adaptation so that
the effect persists. If the rotation of the polarizer is halted, the
visualization fades rapidly. 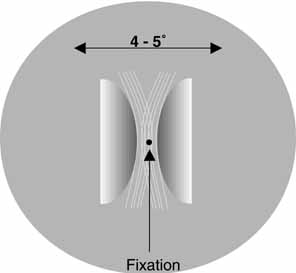 Fig. 4 An illustration of the appearance of Haidinger's brushes in the central
visual field when a source of polarized light is viewed. The images
extend from the fixation point in a pattern similar to a Maltese cross
or a windmill, with the yellow brushes standing out against a blue
background when white light is used. The blue arms coincide with the
electric vector of the polarized light, while the yellow brushes are
oriented perpendicular to the polarity of the source. Fig. 4 An illustration of the appearance of Haidinger's brushes in the central
visual field when a source of polarized light is viewed. The images
extend from the fixation point in a pattern similar to a Maltese cross
or a windmill, with the yellow brushes standing out against a blue
background when white light is used. The blue arms coincide with the
electric vector of the polarized light, while the yellow brushes are
oriented perpendicular to the polarity of the source.
|
Haidinger's brushes apparently are caused by the fact that some structure
of the eye acts as a radial analyzer for blue light. In 1844 Van
Haidinger described the brushes as an entoptic phenomenon caused by
the birefringent properties of the ocular media. Helmholtz assumed that
the yellow pigment overlying the photoreceptors within the macula was
birefringent and could act as a polarizer. This yellow screening pigment
is found within a region of 6 degrees to 10 degrees from the fovea (an
area called macula lutea), and Wald found this pigment
to be the carotenoid xanthophyll with an absorption maximum at 430 to 490 nm. Attempts
to quantify the absorption of blue light by this yellow
pigment have been made by measuring the visibility of Haidinger's
brushes at different wavelengths. DeVries and colleagues27 and Naylor and Stanworth28 both demonstrated that the spectral distribution of the effect was virtually
indistinguishable from the optical density spectrum of the macular
pigment. Although the origin of macular dichroism has not been fully
explained, the radial structure of the Henle fiber layer appears to
play an important role. Bone29 and Bone and Landrum30 suggested that the radially arranged nerve fibers between the inner and
outer limiting membrane of the retina provide a matrix for the alignment
of xanthophyll molecules. Alternatively, Hemenger31 has argued that form dichroism (arising only from the radial structure
of Henle's fiber layer and not from the preferential orientation
of molecules) must contribute to the phenomena. Additionally, the
cornea and lens may have an effect on the polarization of the incoming
light. In particular, the birefringent properties of the collagen
fibrils within the cornea could influence the orientation of the brushes, because
the collagen in the corneal stroma is predominantly oriented
in an upward and outward diagonal.32 This contribution from the preretinal media could explain why patients
with keratoconus have difficulty perceiving this entoptic phenomenon. The visualization of Haidinger's brushes has been applied in several
different clinical contexts. Because the effect is caused by the orientation
of elements in front of the photoreceptors, any process disrupting
this orientation without severely affecting the photoreceptors
or retinal circuitry could lead to a reduction in the visibility of the
brushes while minimally affecting visual acuity. For example, patients
with central serous retinopathy or macular edema may be unable to detect
the brushes at visual acuity levels (20/40 to 20/80) that
exceed the level to which acuity must be reduced by lenticular
opacification to obscure the effect (20/200 to 20/400). The
visualization of Haidinger's brushes also has been used
to determine the angle between fixation and foveal axes in amblyopic
eyes with eccentric fixation. In addition, the phenomenon has been
used in the diagnosis and treatment of binocular suppression scotomata
associated with some forms of esotropia. |