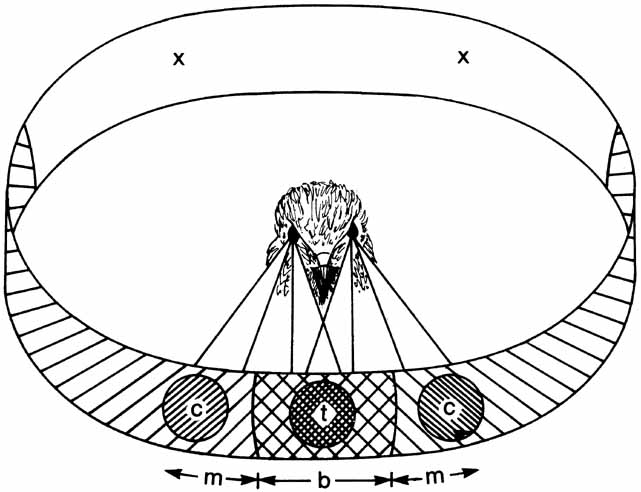
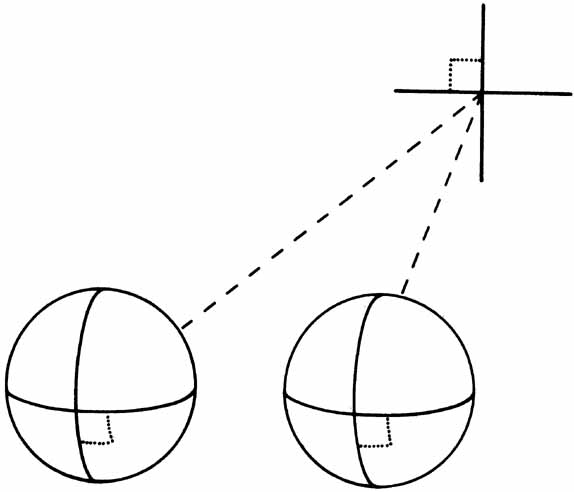
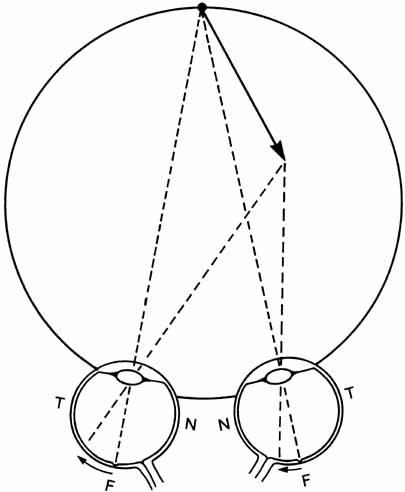
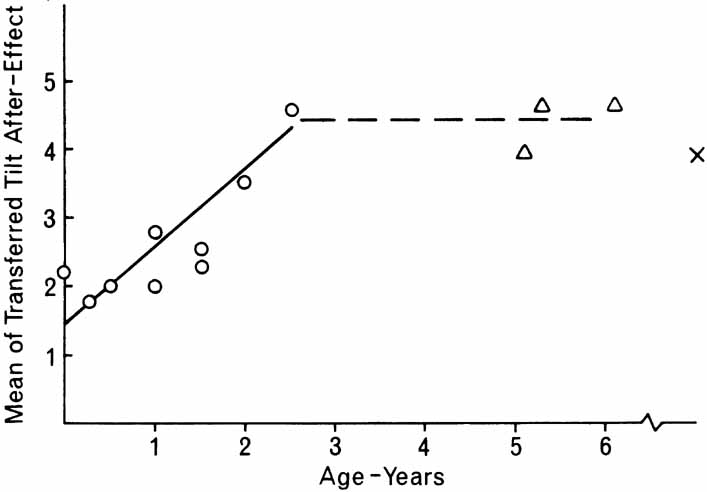
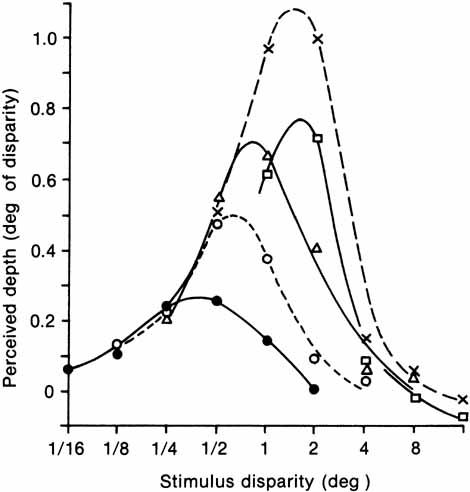
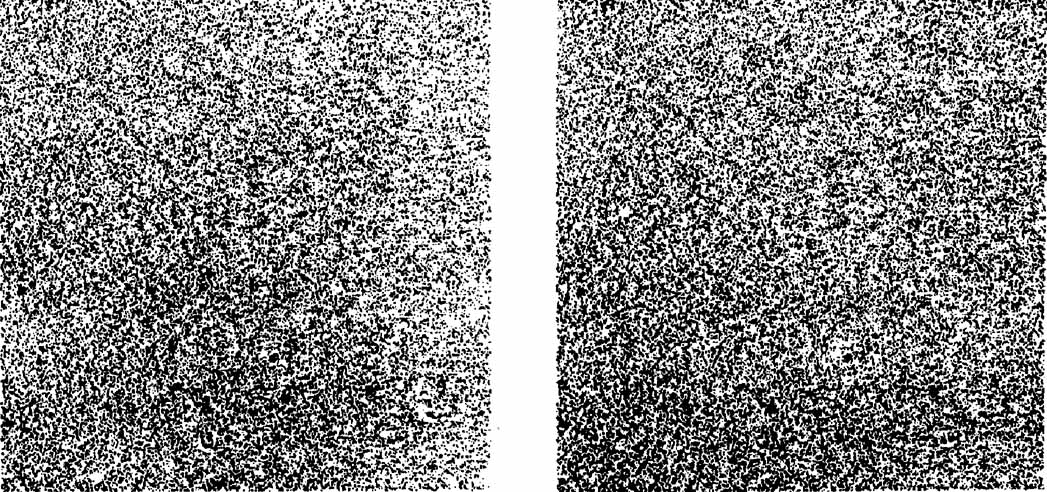
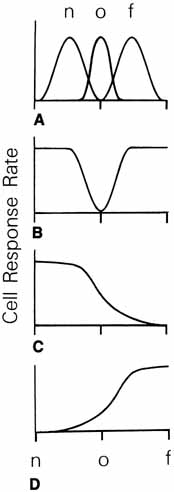
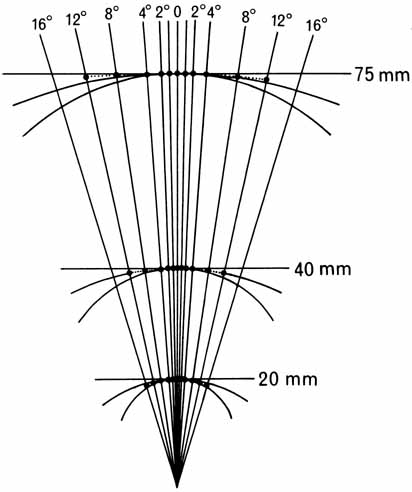
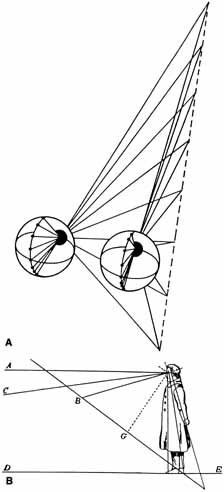
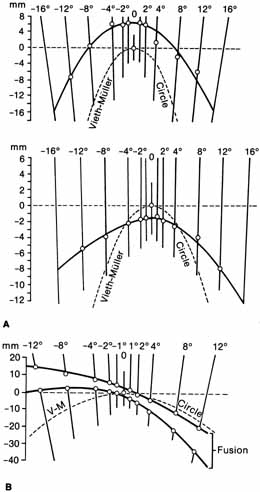
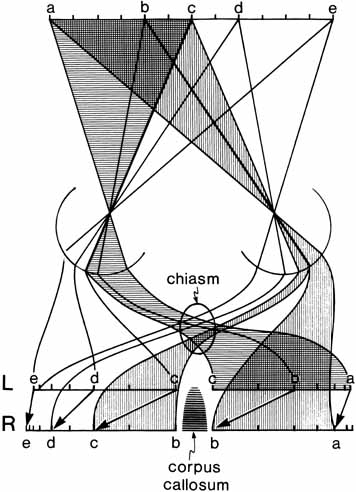
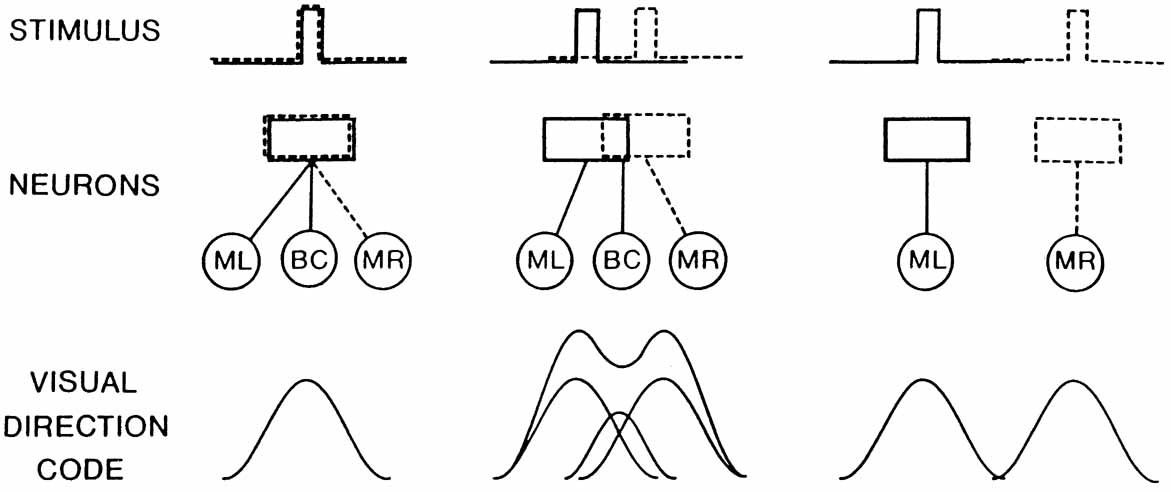
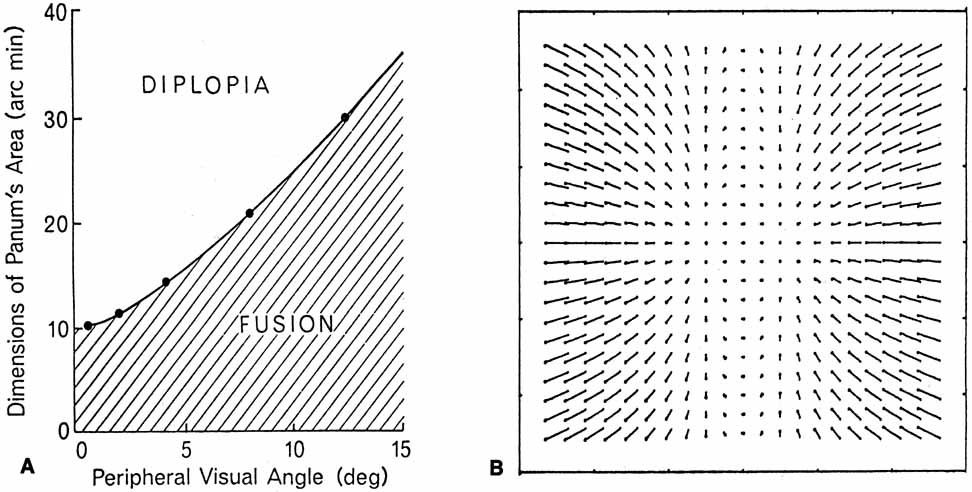
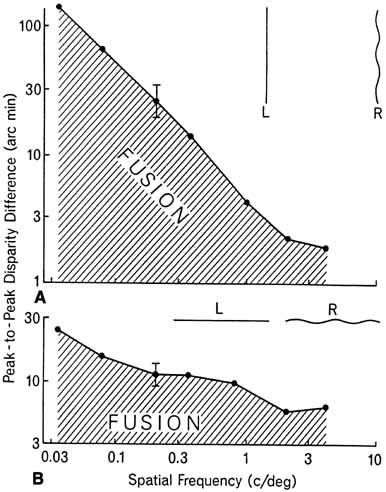
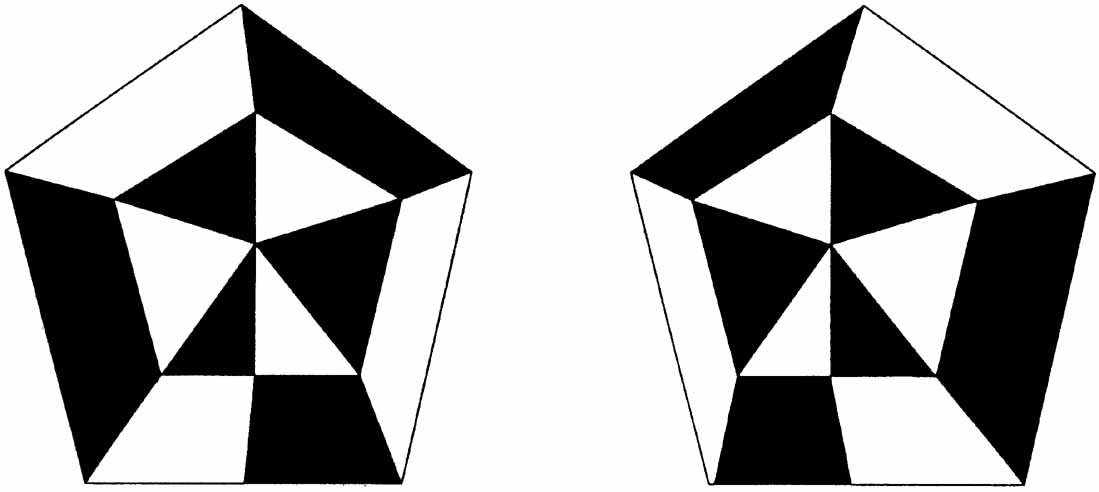
1. Walls GL: The Vertebrate Eye. New York:Hafner, 1967 2. Wheatstone C: Some remarkable phenomena of binocular vision. Philos Trans R Soc Lond 128:374, 1838 3. Bough EW: Stereoscopic vision in the macaque monkey: A behavioral demonstration. Nature 225:42, 1970 4. Fox R, Blake RR: Stereoscopic vision in the cat. Nature 233:55, 1971 5. Fox R, Lehmkuhle SW, Bush RC: Stereopsis in the falcon. Science 197:79, 1976 6. Steinbach MJ, Money KE: Eye movements in the owl. Vision Res 13:889, 1973 7. Wiesel TN, Hubel DH: Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol 158:307, 1974 8. Anker RL, Cragg BG: Development of the extrinsic connections of the visual cortex in the cat. J Comp Neurol 158:307, 1974 9. Blakemore C, Van Sluyters RC: Innate and environmental factors in the development of the kitten's
visual cortex. J Physiol 248:663, 1975 10. Wiesel TN, Hubel DH: Effects of visual deprivation on morphology and physiology of cells in
the cat's lateral geniculate body. J Neurophysiol 26:978, 1963 11. Blakemore C. Garey LJ, Vital-Durand F: The physiological effects of monocular deprivation and their reversal in
the monkey's visual cortex. J Physiol 283:223, 1978 12. LeVay S, Wiesel TN, Hubel DH: The development of ocular dominance columns in normal and visually deprived
monkeys. J Comp Neurol 191:1, 1980 13. Crawford LJ, De Faber JT, Harwerth RS, et al: The effects of reverse monocular deprivation in monkeys: II. Electrophysiological
and anatomical studies. Exp Brain Res 74:338, 1989 14. Harwerth RS, Smith EL, Crawford mLJ, von Noorden GK: Behavioral studies of the sensitive period of development of visual functions
in monkeys. Behav Brain Res 41:179, 1990 15. Wiesel TN, Hubel DH: Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol 28:1060, 1965 16. Swindale NV, Vital-Durand F, Blakemore C: Recovery from monocular deprivation in the monkey: III. Reversal of anatomical
effects in the visual cortex. Proc R Soc Lond 213:435, 1981 17. Bakemore C, Cooper GF: Development of the brain depends on the visual environment. Nature 228:477, 1970 18. Hirsch HVB, Spinelli DN: Visual Experience modifies distribution of horizontally and vertically
oriented receptive fields in cats. Science 168:869, 1970 19. Hubel DH, Wiesel TN: Binocular interaction in striate cortex of kittens reared with artificial
squint. J Neurophysiol 28:1041, 1965 20. Spinelli DN, Hirsch HVB, Phelps J, Metzler T: Visual experience as a determinant of the response characteristics of cortical
receptive fields in cats. Exp Brain Res 15:289, 1972 21. Smith EL, Harwerth RS, Crawford mLJ: Spatial contrast sensitivity deficits in monkeys produced by optically
induced danisometropia. Invest Ophthal Vis Sci 26:330, 1985 22. Blakemore C: Developmental factors in the formation of feature extracting neurons. In Schmidt FO, Worden FG (eds). The Neurosciences: Third Study Program. Cambridge: MIT Press, 1974:105–113 23. Garey LJ, Fisken RA, Powell TPA: Effects of experimental deafferentation on cells in the lateral geniculate
nucleus of the cat. Brain Res 52:363, 1973 24. Chow K, Stewart D: Reversal of structural and functional effects of long-term visual
deprivation in cats. Exp Neurol 34:409, 1972 25. Cleland BG, Dubin MW, Levick WR: Sustained and transient neurons in the cat's retina and lateral geniculate
nucleus. J Physiol 217:473, 1971 26. Sherman SM, Hoffman KP, Stone J: Loss of a specific cell type from the dorsal lateral geniculate nucleus
in visually deprived cats. J Neurophysiol 35:532, 1972 27. Hubel DH, Wiesel TN: The period of susceptibility to the physiological effects of unilateral
eye closure in kittens. J Physiol 206:419, 1970 28. Wiesel TN, Hubel DH: Single cell responses in striate cortex of kittens deprived of vision in
one eye. J Neurophysiol 26:1003, 1963 29. Wiesel TN, Hubel DH: Single cell responses in striate cortex of kittens deprived of vision in
one eye. J Neurophysiol 26:1003, 1963 30. Wiesel TN, Hubel DH: Comparison of the effects of unilateral and bilateral eye closure on cortical
unit responses in kittens. J Neurophysiol 28:1029, 1965 31. Blakemore C, Mitchell DE: Environmental modification of the visual cortex and the neural basis of
learning and memory. Nature 241:467, 1973 32. Shlaer R: Shift in binocular disparity causes compensatory change in the cortical
structure of kittens. Science 173:638, 1971 33. Pettigrew JD: The importance of early visual experience for neurons of the developing
geniculostriate system. Invest Ophthal 11:386, 1972 33a. Nixon RB, Helveston EM, Miller K, Archer SM, Ellis FD: Incidence of strabismus in neonates. Am J Ophthalmol 100, 798, 1985 34. Held R, Birch EE, Gwiazda J: Stereoacuity of human infants. Proc Natl Acad Sci USA 77:5572, 1980 35. Birch EE, Gwiazda J, Held R: Stereoacuity development for crossed and uncrossed disparities in human
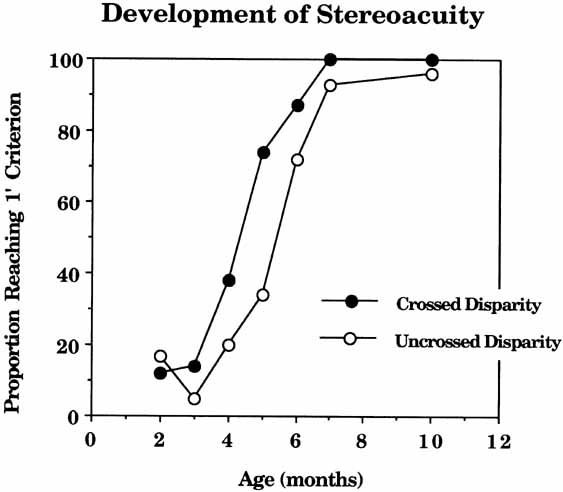
infants. Vision Res 22:507, 1982 36. Birch E, Petrig B: FPL and VEP measures of fusion, stereopsis and stereoacuity in normal infants. Vision Res 36:1321–1327, 1996 37. Birch RR, Hale LA: Operant assessment of stereoacuity. Clin Vision Sci 4:295, 1989 38. Romano PE, Romano JA, Puklin JE: Stereoacuity development in children with normal binocular single vision. Am J Ophthal 79:966, 1975 39. Simons K: Stereoacuity norms in young children. Arch Ophthamol 99:439, 1981 40. Tatsumi S, Tahira K: Study on the stereotest (Titmus). Folia Ophthal Jpn 23:620, 1972 41. Leguire LE, Rogers GL, Bremer DL: Visual-evoked response binouclar summation in normal and strabismic
infants. Invest OphthalVis Sci 32:126, 1991 42. Braddick Oj, Atkinson J, Julesz B, et al: Cortical Binocularity in Infants. Nature 288:363, 1980 43. Petrig B, Julesz B, Kropfl W, et al: Development of stereopsis and cortical binocularity in human infants: Electrophysiological
evidence. Science 213:1402, 1981 44. Taylor DM: Is congenital esotropia functionally incurable? Trans Am Ophthal Soc 70:529, 1973 45. Ing MR: Early surgical alignment for congenital esotropia. J Pediatr Ophthal Strabismus 20:11, 1983 46. Dellar M: Why should surgery for early onset strabismus be postponed? Br J Ophthal 72:100, 1988 47. Richards W: Stereopsis and stereo blindness. Exp Brain Res 10:380, 1970 48. Mitchell DE, Reardon J, Muir DW: Interocular transfer of the motion after-effect in normal and stereoblind
observers. Exp Brain Res 22:163, 1975 49. Hubel DH, Wiesel TN: Aberrant visual projection in the Siamese cat. J Physiol 218:33, 1971 50. Cool ST, Crawford mLJ: Absence of binocular coding in striate cortex of Siamese cats. Vision Res 12:1809, 1972 51. Apkarian P, Spekreijse H: The VEP and misrouted pathways in human albinism. In Cracco RQ, Bodis-Wolner I (eds): Evoked Potentials. New York: Alan R Liss, 1986:211–226 52. Wade NJ: On interocular transfer of the movement after-effect in individuals
with and without normal binocular vision. Perception 5:113, 1976 53. Hohmann A, Creutzfeldt OD: Squint and the development of binocularity in humans. Nature 254:613, 1975 54. Banks MS, Aslin RN, Letson RD: Sensitive period for the development of human binocular vision. Science 190:675, 1975 55. Romano PE: Pediatric ophthalmic mythology. Postgrad Med 58:146, 1975 56. Taylor DM: Is congenital esotropia functionally curable? Trans Am OphthalSoc 70:529, 1972 57. Levi DM: Spatial vision in amblyopia. In Cronly-Dillon JR (ed): Vision and Visual Disorders, vol 10. 1991:212–238 58. Worth CA, Chavasse FB: In Lyle TK (ed). Squint, Its Causes, Pathology and Treatment, 8th ed. Philadelphia: Blackston, 1950 59. von Noorden GK: Burian-von Noorden's Binocular Vision and Ocular Motility. St. Louis: CV Mosby, 1980 60. Day SH, Orel-Bixler DA, Norcia AM: Abnormal acuity development in infantile esotropia. Invest Ophthal Vis Sci 29:165, 1988 61. Cuppers C: Grenzen and Moglichkeit der pleoptischen Therapie. In Hollowich F (ed). Schielen: Pleoptik Orthopit, Operation. Stuttgart: Ferdinance Enke Verlag, 1961:1–68 62. Broendstrup P: Amblyopia ex anopsia in infantile cataract. Acta Ophthal 22:52, 1944 63. Freeman RD, Mitchell DE, Millodot M: A neural effect of partial visual deprivation in humans. Science 175:1384, 1972 64. von Noorden GK, Ryan SJ, Maumanee AE: Management of congenital cataract. Trans Am Acad Ophthal Otolaryngol 4:352, 1970 65. Mustari M, Cynader M: Prior strabismus protects kitten cortical neurons from the effects of monocular
deprivation. Brain Res 211:165, 1981 66. Smith EL III , Harwerth RS, Siderov J, et al: Prior binocular dissociation reduces monocular form deprivation amblopia
in monkeys. Invest Ophthal Vis Sci 33:1804, 1992 67. Gottlob I, Stangler-Zuschrott E: Effect of levodopa on contrast sensitivity and scotoma in human amblyopia. Invest Ophthal Vis Sci 31:776, 1990 68. Gottlob I, Charlier J, Reinecke RD: Visual acuities and scotomas after one week levodopa administration in
human amblyopia. Invest Ophthal Vis Sci 33:2722, 1992 69. Leguire LE, Rogers GL, Bremer DL, et al: Longitudinal study of levodopa/carbidopa for childhood amblyopia. J Pediatr Ophthal Strab 354, 30, 1993 70. Rogers BJ, Glennerster A, Bradshaw MF: Does the visual system use vertical disparities or vertical size ratios
for depth and size scaling? Proc ECVP 6, 1993 71. Howard HJ: A test for the judgment of distance. Am J Ophthal 2:656, 1919 72. Hecht S, Mintz EU: The visibility of single lines at various illuminations and the retinal
basis of visual resolution. J Gen Physiol 22:593, 1939 73. Ogle KN, Ellerbrock VJ: Stereoscopic sensitivity in the space eikonometer. Arch Ophthal 34:303, 1945 74. Richards W, Kaye MG: Local versus global stereopsis: Two mechanisms? Vision Res 14: 1345, 1974 75. Tyler CW: Stereoscopic vision: Cortical limitations a disparity scaling effect. Science 181:276, 1973 76. Tyler CW: Spatial organization of binocular disparity sensitivity. Vision Res 15:583, 1975 77. Mitchell DE, O'Hagan S: Accuracy of stereoscopic localization of small line segments that differ
in size and orientation for the two eyes. Vision Res 12:437, 1972 78. Julesz B: Binocular depth perception of computer generated patterns. Bell Syst Tech J 39:1125, 1960 79. Ogle KN: On the limits of stereoscopic vision. J Exp Psychol 44–253, 1952 80. Julesz B: Foundations of Cyclopean Perception. Chicago: University of Chicago Press, 1971 81. Barlow HB, Blakemore C, Pettigrew JD: The neural mechanism of binocular depth discrimination. J Physiol 193:327, 1967 82. Hubel DH, Wiesel TN: Cells sensitive to binocular depth in area 18 of the macaque monkey cortex. Nature 225:41, 1970 83. Joshua DE, Bishop PO: Binocular single vision and depth discrimination. Exp Brain Res 10:389, 1970 84. Pettigrew JD, Nikara T, Bishop PO: Binocular interactions of single units in cat striate cortex: Stimulation
by single moving slit. Exp Brain Res 6:391, 1968 85. Von der Heydt R, Adorjani C, Hanny P, Baumgartner G: Disparity sensitivity and receptive field incongruity of units in the cat
striate cortex. Exp Brain Res 31:523, 1978 86. Poggio CF, Fischer B: Binocular interaction and depth sensitivity neurons in striate and prestriate
cortex of the behaving rhesus monkey. J Neurophysiol 40:1392, 1977 87. Blakemore C, Fiorentini A, Maffei L: A second neural mechanism of binocular depth discrimination. J Physiol 226:725, 1972 88. Koenderink JJ, van Doorn AJ: Geometry of binocular vision and a model for stereopsis. Biol Cybern 21:29, 1976 89. Pettigrew JD: Binocular neurons which signal change of disparity in area 18 of cat visual
cortex. Nature 214:123, 1973 90. Regan DM, Cynader M: Neurons in cat parastriate cortex sensitive to direction of motion in three-dimensional
space. J Ophthal 81(Suppl), 1977 91. Fiorentini A, Maffei L: Electrophysiological evidence for binocular disparity detectors in human
visual system. Science 169:208, 1970 92. Srebro R: The visually evoked response: Binocular facilitation and failure when binocular
vision is disturbed. Arch Ophthal 96:839, 1978 93. Apkarian P, Levi D, Tyler CW: Binocular facilitation in the visual-evoked potential of strabismic
amblyopes. Am J Optom Physiol Opt 58:820, 1981 94. Apkarian P, Nakayama K, Tyler CW: Binocularity in the human visual evoked potential: facilitation, summation
and suppression. Electroenceph Clin Neurophysiol 51:32, 1981 95. Regan D, Spekreijse H: Electrophysiological correlate of binocular depth perception in man. Nature 225:92, 1970 96. Lehmann D, Julesz B: Lateralized cortical potentials evoked in humans by dynamic random-dot
stereograms. Vision Res 18:1265, 1978 97. Julesz B, Kropfl W, Petrig B: Large evoked potentials to dynamic random-dot correlograms permit
quick determination stereopsis. Proc Natl Acad Sci USA 77:2348, 1980 98. Norcia AM, Tyler CW: Temporal frequency limits for stereoscopic apparent motion processes. Vision Res 24: 395, 1984 99. Aguilonius F: Opticorum Libri Sex. Antwerp, Plantin, 1613 100. Vieth GAW: Ueber die Richtung der Augen. Ann Phys 48:233, 1818 101. Müller J: Vom Gesichtsinn. In Handbuch der Physiologie des Menschen fur Vorlesungen. Holscher: Coblenz 1840 102. See Meyer: Handbuch der Physiologie. Berlin 1833 103. Prevost A: Essaui sur la Theorie de la Vision Binoculaire. Geneva, Ramboz, 1843 104. von Helmholtz H: Handbuch der Physiologische Optik. Hamburg: Voss, 1866 105. Hering E: Beitrage zur Physiologie. Leipzig: W. Engelman, 1864 106. Hillebrand F: Die stabilitat der Raumwerte auf der Netzhaut. Z Psychol 5:1, 1893 107. Oglie KN: Researches in Binocular Vision. Philadelphia: WB Saunders, 1950 108. Ogle KN: Analytical treatment of the longitudinal horopter. J Opt Soc Am 22:665, 1932 109. Voklmann AW: die Stereoskopischen Erscheinungen in ihrer Beziehung zu der Lehre von
den identischen Netzhautpunkten. Graefes Arch Ophthal 2:1, 1859 110. Schor CM: Fixation disparity and vergence adaptation. In Schor CM, Cuiffreda KJ. Vergence Eye Movements: Basic and Clinical Aspects. Boston: Butterworths, 1983:465–516 111. Peters HB: The influence of anisometropia on stereosensitivity. Am J Opt 46:120, 1969 112. Bagolini B, Capobianco NM: Subjective space in comitant squint. Am J Ophthal 59:430, 1965 113. McKee SP, Levi DM, Movshon JA: The pattern of visual deficits in amblyopia. J Vis 3(5):380–405, 2003 114. Thompson AM, Nawrot M: Depth perception from motion parallax in amblyopic observers. Vision Res 39:1407–1413, 1999 115. Levi DM, Klein SA, Wang H: Discrimination of position and contrast in amblyopic and peripheral vision. Vision Res 34(24):3293–3313, 1994 116. Levi DM, Klein SA, Wang H: Amblyopic and peripheral vernier acuity: a test-pedestal approach. Vision Res 34(24):3265–3292, 1994. Check 1994a,b 117. Levi DM, Klein SA: Limitations on position coding imposed by undersampling and univariance. Vision Res 36(14):2111–2120, 1996 118. Field DJ, Hess RF: Related Articles, Uncalibrated distortions vs undersampling. Vision Res 36(14):2121–4, 1996 119. Wang H, Levi DM, Klein SA: Spatial uncertainty and sampling efficiency in amblyopic position acuity. Vision Res 38(9):1239–1251, 1998 120. Mussap AJ, Levi DM: Orientation-based texture segmentation in strabismic amblyopia. Vision Res 39(3):411–418, 1999 121. Levi DM, Klein SA, Sharma V: Position jitter and undersampling in pattern perception. Vision Res 39(3):445–465, 1999 122. Sharma V, Levi DM, Coletta NJ: Sparse-sampling of gratings in the visual cortex of strabismic amblyopes. Vision Res. 39(21):3526–3236, 1999 123. Norcia AM, Harrad RA, Brown RJ: Changes in cortical activity during suppression in stereoblindness. Neuroreport. 11(5):1007–1012, 2000 124. Tomac S, Birdal E: Effects of anisometropia on binocularity. J Pediatr Ophthal Strabismus. 38(1):27–33, 2001 125. Weakley DR Jr : The association between nonstrabismic anisometropia, amblyopia, and subnormal
binocularity. Ophthalmology 108(1):163–71, 2001 126. Richard JM, Parks MM: Intermittent exotropia. Surgical results in different age groups. Ophthalmology. 90(10):1172–1177, 1983 127. Bateman JB, Parks MM, Wheeler N: Discriminant analysis of acquired esotropia surgery. Predictor variables
for short- and long-term outcomes. Ophthalmology 90(10):1154–9, 1983 128. Helveston EM, Ellis FD, Schott J, Mitchelson J, Weber JC, Taube S, Miller K: Surgical treatment of congenital esotropia. Am J Ophthalmol 96(2):218–28, 1983 129. O'Keefe M, Abdulla N, Bowell R, Lanigan B: Binocular function and amblyopia after early surgery in infantile eosotropia. Acta Ophthal Scand 74(5):461–2, 1996 130. Bateman JB, Parks MM, Wheeler N. Discriminant analysis of congenital esotropia surgery. Predictor variables
for short- and long-term outcomes. Ophthalmology 90(10):1146–53, 1983 131. Tytla ME, Lewis TL, Maurer D, Brent HP: Stereopsis after congenital cataract. Invest OphthalVis Sci 34(5):1767–73, 1993 132. Wright KW, Matsumoto E, Edelman PM: Binocular fusion and stereopsis associated with early surgery for monocular
congenital cataracts. Arch Ophthalmol 110(11):1607–9, 1992 133. Jeffrey BG, Birch EE, Stager DR Jr , Stager DR Sr , Weakley DR Jr : Early binocular visual experience may improve binocular sensory outcomes
in children after surgery for congenital unilateral cataract AAPOS 5(4):209–16, 2001 134. Panum PL: Physiologische Untersuchungen uber das Sehen mit zwei Augen. Kiel: Schwering, 1858 135. Verhoeff FH: A new theory of binocular vision. Arch Ophthal 13:11, 1935 136. Roenne G: The physiological basis of sensory fusion. Acta Ophthal 344:1, 1956 137. Hubel DH, Wiesel TN: Receptive fields, binocular interaction and functional architecture in
the cat' visual cortex. J Physiol 160:106, 1962 138. Linksz A: The horopter: An analysis. Trans Am Ophthal Soc 52:877, 1954 139. Woo GCS: The effect of exposure time on the foveal size of Panum's area. Vision Res 14:473, 1974 140. Julesz B, Tyler CW: Neurontropy: An Entropy-like measure of neural correlation in binocular
fusion and rivalry. Biol Cybernetics 23:25, 1976 141. Gouras P, Armington JC, Dropfl WJ, Gunkel RD: Electronic computation of human retinal and brain responses to light stimulation. Ann NY Acad Sci 115:763, 1964 142. Perry NW, Childers DG, McCoy JG: Binocular addition of the visual evoked responses to pattern stimuli in
humans. Vision Res 8:547, 1968 143. Harter MR, Seiple WH, Salmon L: Binocular summation of visually evoked responses to pattern stimuli in
Humans. Vision Res 13:1433, 1973 144. Campbell FW, Maffei L: Electrophysiological evidence for the existence of orientational and size
detectors in the human visual system. J Physiol 207:635, 1970 145. Spekreijse H: Analysis of EEG Responses in Man. The Hague: Junk, 966 146. Tyler CW, Apkarian P, Levi DM, Nakayama K: Rapid assessment of visual function: An electronic sweep technique for
the pattern VEP. Invest Ophthal Vis Sci 18:703, 1979 147. Hering E: Outlines of a Theory of the light Sense. Cambridge, MA:. Harvard Univ Press, 1920 (trans. 1964) 148. Wolfe JM: Influence of spatial frequency, luminance and duration on binocular rivalry
and abnormal fusion of briefly presented dichoptic stimuli. Perception 12:447, 1983 149. Liu L, Tyler CW, Schor CM: Failure of rivalry at low contrast: Evidence of a suprathreshold binocular
summation process. Vision Res 32:1471, 1992 150. Baizer JS, Robinson DL, Dow BM: Visual responses of area 18 neurons in awake behaving monkey. J Neurophysiol 40:1024, 1977 151. Blake R, Fox R, McIntyre C: Stochastic properties of stabilized-image binocular rivalry alternations. J Exp Psychol 88:327, 1971 152. Bárány EH, Hallden U: The influence of some central nervous system depressants on the reciprocal
inhibition between the two retinas as manifested in retinal rivalry. Acta Psychol Scand 14:296, 1947 153. Lack LC: The role of accommodation in the control of binocular rivalry. Percept Psychophys 10:38, 1971 154. Levelt WJM: On binocular rivalry. Institute for Perception, RVO/TNO: Soesterberg, 1965 155. Fox R, Check R: Binocular fusion: A test of the suppression theory. Percept Psychophys 1:331, 1966 156. Collter SC, Bevan W: Objective measurement of dominance control in binocular rivalry. Percept Psychophys 8:437, 1970 157. Fox R, Check R: Detection of motion during binocular rivalry suppression. J Exp Psychol 78:388, 1968 158. Wales R, Fox R: Increment detection thresholds during binocular rivalry suppression. Percept Psychophys 8:90, 1970 159. Fox R, Check R: Forced choice recognition of form during binocular rivalry. Psychon Sci 6:471, 1966 160. Fox R, Check R: Independence between binocular rivalry duration and magnitude of suppression. J Exp Psychol 93:283, 1972 161. Blake R, Fox R: Binocular rivalry suppression: Insensitive to spatial frequency and orientation
change. FRVision Res 14:687, 1974 162. Breese BB: On inhibition. Psychol Monogr 3:1, 1899 163. Kakizaki S: Binocular rivalry and stimulus intensity. Jpn Psychol Res 2:94, 1960 164. Fox R, Rasche F: Binocular rivalry and reciprocal inhibition. Percept Psychophys 5:215, 1969 165. Lehmkuhle SW, Fox R: Effect of binocular suppression on the motion aftereffect. Vision Res 15:855, 1975 166. Blake R, Fox R: Adaptation to invisible gratings and the site of binocular rivalry suppression. Nature 249:488, 1974 167. Cobb WA, Morton HB, Ettlinger G: Cerebral potentials evoked by pattern reversal and their suppression in
visual rivalry. Nature 216:1123, 1967 168. Van der Tweel LH, Spekrijse H, Regan D: A correlation between evoked potentials and point-to-point
interocular suppression. Electroencephologr Clin Neurophysiol 28:209, 1970 |