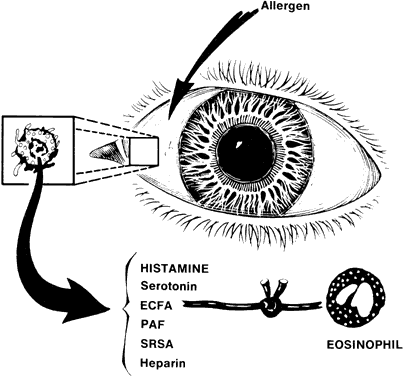
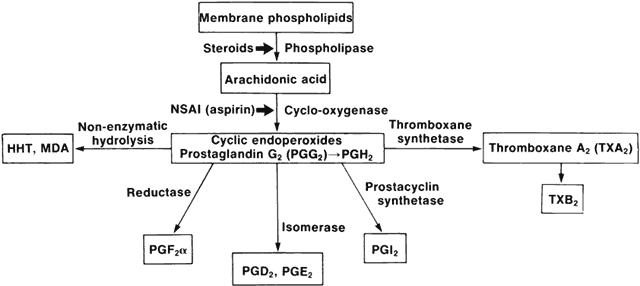
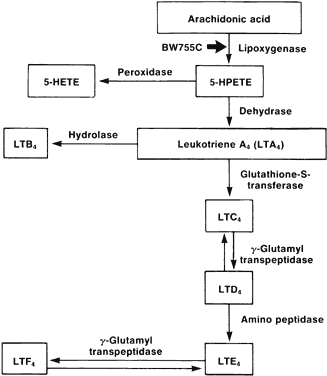
1. Metzger H, Bach MK: The receptors for IgE in mast cells and basophils: Studies
on IgE binding and on the structure of the receptor. In Bach MK (ed): Immediate
Hypersensitivity: Modern Concepts Developments, p 561. New
York, Dekker, 1978 2. Allansmith MR: Immunology of the eye. In: The Eye and Immunology, p 99. St. Louis, CV
Mosby, 1982 3. Feldberg W: Distribution of histamine in the body. In Wolstenholme GEW, O'Connor
CM (eds): Histamine (CIBA Foundation Symposium). Boston, Little, Brown & Co, 1956 4. Riley JR: The effects of histamine-liberators on the mast cells of the rat. J Pathol Bacteriol 65:471, 1953 5. Humphery JH, Jacques R: The release of histamine and 5-hydroxytryptamine (serotonin) from platelets
by antigen-antibody reactions (in vitro). J Physiol 128:9, 1955 6. Graham HT, Wheelwright F, Parich HH et al: Distribution of histamine among the blood elements. Fed Proc 11:350A, 1952 7. Lewis RA, Goetzl EJ, Wasserman SI et al: The release of four mediators of immediate hypersensitivity from human
leukemic basophils. J Immunol 114:87, 1975 8. Beaven MA: Histamine. Its role in physiological and pathological processes. Monogr Allergy 13:1, 1978 9. Udell IJ, Abelson MB: Animal and human ocular surface response to a topical nonimmune mast-cell
degranulating agent (compound 48/80). Am J Ophthalmol 91:226, 1981 10. Udell JJ, Kenyon KR, Hanninen L et al: Conjunctival mast cell degranulation in compound 48/80 model (abstr). Invest Ophthalmol Vis Sci 20(suppl):9, 1981 11. Caulfield JP, Lewis RA, Hein A et al: Secretion in dissociated human pulmonary mast cells. J Cell Biol 85:299, 1980 12. Dale HH, Laidlaw PP: Histamine shock. J Physiol 52: 355, 1919 13. Lewis T: The blood vessels of the human skin and their responses. London, Shaw & Sons, 1927 14. Keele CA, Armstrong D: Substances Producing Pain and Itch. Baltimore, Williams & Wilkins, 1964 15. Robertson I, Greaves MW: Responses of human skin blood vessels to synthetic histamine analogues. Br J Clin Pharmacol 5:319, 1978 16. Harvery RP, Schocket AL: The effect of H1 and H2 blockade on cutaneous histamine response in man. J Allergy Clin Immunol 65:136, 1980 17. McCusker MT, Chung KF, Robert NM, Barnes PJ: Effects of topical capsaicin on the cutaneous responses to inflammatory
mediators and to antigen in man. J Allergy Clin Immunol 83:1118, 1989 18. Smith JA, Mansfield LE, deShazo R, Nelson HS: An evaluation of the pharmacologic inhibition of the immediate and late
cutaneous effects to allergen. J Allergy Clin Immunol 65:118, 1980 19. Abelson MB, Schaefer K: Conjunctivitis of allergic origin: Immunologic mechanisms and current approaches
to therapy. Surv Ophth 38:115, 1993 20. Abelson MB, Allansmith MR: Histamine and the eye. In Silverstein AM, O'Connor
GR (eds): Immunology and Immunopathology of the Eye, pp 362–364. New
York, Masson, 1979 21. Weston JH, Udell IJ, Abelson MB: H1 receptors in the human ocular surface (abstr). Invest Ophthalmol Vis Sci 20(suppl):32, 1981 22. Abelson MB, Udell IJ: H2 receptors in the human ocular surface. Arch Ophthalmol 99:302, 1981 23. Abelson MB, Baird RS, Allansmith MR: Tear histamine levels in vernal conjunctivitis and other ocular inflammations. Ophthalmology 87:812, 1980 24. Abelson MB, Soter N, Simon M et al: Histamine in human tears. Am J Ophthalmol 85:417, 1977 25. Henriquez AS, Kenyon KR, Allansmith MR: Mast cell ultrastructure: Comparison in contact lens-associated giant papillary
conjunctivitis and vernal conjunctivitis. Arch Ophthalmol 99:1266, 1981 26. Allansmith MR, Baird RS, Greiner JV: Vernal conjunctivitis and contact-lens associated giant papillary conjunctivitis
and vernal conjunctivitis. Arch Ophthalmol 99:884, 1981 27. Berdy GJ, Levene RB, Bateman ST et al: Identification of histaminase activity in human tears after conjunctival
antigen challenge. Invest Ophthalmol Vis Sci 31 (ARVO suppl):65, 1990 28. Abelson MB, Leonardi AA, Smith LM et al: Histaminase activity in patients with vernal keratoconjunctivitis. Ophthalmology 102(12):1958, 1995 29. Schwartz LB: Mediators of human mast cells and human mast cell subsets. Ann Allerg 58:226, 1987 30. Reiss J, Abelson MB, George MA, Wedner JH: Allergic Conjunctivitis. In
Pepose J, Holland G, Wilhelmus K (eds): Ocular Infection and Immunity, p 347. Boston, Mosby, 1996 31. Butrus SI, Ochsner KI, Abelson MB, Schwartz LB: The level of tryptase in human tears: An indicator of activation of conjunctival
mast cells. Ophthalmology 97:1678, 1990 32. Margrini L, Bonini S, Centofanti M et al: Tear tryptase levels and allergic conjunctivitis. Allergy 51:577, 1996 33. Bonini S, Schiavone M, Bonini S et al: Efficacy of lodoxamide eye drops on mast cells and eosinophils after allergen
challenge in allergic conjunctivitis. Ophthalmology 104: 849, 1997 34. Fukagawa K, Saito H, Azuma N et al: Histamine and tryptase levels in allergic conjunctivitis and vernal keratoconjunctivitis. Cornea 13:345, 1994 35. Schwartz LB, Metcalfe DD, Miller JS et al: Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis
and mastocytosis. N Engl J Med 316:1622, 1987 36. Wenzel S, Irani AA, Sander JM et al: Immunoassay of tryptase from human cells. J Immunol Methods 80:139, 1986 37. Castells M, Schwartz LB: Tryptase levels in nasal-lavage fluid as an indicatory of the immediate
allergic response. J Allergy Clin Immunol 82:348, 1988 38. Schwartz LB, Atkins PC, Bradford TR et al: Release of tryptase together with histamine during the immediate cutaneous
response to allergen. J Allergy Clin Immunol 141:821, 1988 39. Gruber BL, Schwartz LB, Rammamurthy NS et al: Activation of latent rheumatoid synovial collagenase by human mast cell
tryptase. J Immunol 140:3936, 1988 40. Deleuran B, Kristensen M, Larsen CG et al: Increased tryptase levels in suction-blister fluid from patients with urticaria. Br J Dermatol 125:14, 1991 41. Tam EK, Caughey GH: Degradation of airway neuropeptides by human lung tryptase. Am J Respir Cel Mol Biol 3:27, 1990 42. Schechter NM, Choi JK, Slavin DA et al: Identification of a chymotrypsin-like proteinase in human mast cells. J Immunol 137:962, 1986 43. Cahoalon L, Lider O, Schor H et al: Heparin disaccharides inhibit tumor necrosis factor-α production by
macrophages and arrest immune inflammation in rodents. Int Immunol 9:1517, 1997 44. Ingber A, Trattner A, Cohen IR, Mekori YA: Low doses of low-molecular-weight heparin in vivo inhibits the elicitation of contact hypersensitivity. Acta Derm Venereol 7:454, 1994 45. Anderson W, Chan CC, Nussenblatt RB, Whitcup SM: Topical heparin inhibits compound 48/80 induced allergic conjunctivitis. Invest Ophthalmol Vis Sci 35:1291, 1994 46. Kay AB, Stechschult DJ, Austen KF: An eosinophil leukocyte chemotactic factor of anaphylaxis. J Exp Med 83: 602, 1971 47. Kay AB, Austen KF: The IgE-mediated release of an eosinophil leukocyte chemotactic factor
from human lung. J Immunol 107:899, 1971 48. Wasserman SI, Goetzl EJ, Austen KF: Preformed eosinophil chemotactic factor of anaphylaxis (ECF-A). J Immunol 112:351, 1974 49. Kaliner M, Wasserman SI, Austen KF: Immunologic release of chemical mediators from human nasal polyps. N Engl J Med 289:277, 1973 50. Goetzl EJ, Austen KF: Purification and synthesis of eosinophilotactic tetrapeptides of human
lung tissue: Identification as eosinophil chemotactic factor of anaphylaxis (ECF-A). Proc Natl Acad Sci USA 72:4123, 1975 51. Butrus SI, Abelson MB, Allansmith MR: Ocular allergic disorders. In Lockey
RI, Bukantz SG (eds): Principles of Immunology and Allergy, p 166. Philadelphia, WB
Saunders, 1987 52. Ward PA. Chemotaxis of human eosinophils. Am J Pathol 54:121, 1969 53. Wissler JH, Sorkin E, Stecher VJ: Regulation of serum derived chemotactic
activity by the leukotactic binary peptide system. In Sorkin E (ed): Antibiotics
and Chemotherapy, Vol. 19, p 442. Basel, Karger, 1974 54. Wasserman SI, Goetzl EJ, Ellman L et al: Tumor associated eosinophilotactic factor. N Engl J Med 290:420, 1974 55. Wasserman SI, Whitmer D, Goetzl EJ et al: Chemotactic deactivation of human eosinophils by the eosinophil chemotactic
factor of anaphylaxis. Proc Soc Exp Biol Med 148: 301, 1975 56. Zeiger RS, Yurdin D, Colten HR: Histamine metabolism. II. Cellular and subcellular localization of the
catabolic enzymes, histaminase and histamine methyl transferase in human
leukocytes. J Allergy Clin Immunol 58:172, 1976 57. Lee D: Antihistamine activity of the eosinophil. J Pathol 99:96, 1969 58. Nilzen A: The antihistamine effect of human eosinophils. Allerg Asthmaforsch 16:24, 1970 59. Kater LA, Goetzl EJ, Austen KF: Isolation of human eosinophil phospholipase D. J Clin Invest 57:1173, 1976 60. Wasserman SI, Goetzl EJ, Austen KF: Inactivation of slow reacting substance of anaphylaxis by human eosinophil
arylsulfatase. J Immunol 114:645, 1975 61. Wasserman SI, Goetzel EJ, Austen KF: Inactivation of human SRS-A by intact human eosinophils and by eosinophil
arylsulfatase (abstr). J Allergy Clin Immunol 55:72, 1975 62. Orange RP, Murphy RC, Austen KF: Inactivation of slow reacting substance of anaphylaxis (SRS-A) by arylsulfatases. J Immunol 113:316, 1974 63. Butterworth AE, David JR: Current concepts: Eosinophil function. N Engl J Med 304:154, 1981 64. Gleich GJ, Loegering DA, Maldonado JE: Identification of a major basic protein in guinea pig eosinophil granules. J Exp Med 137:1459, 1973 65. Archer GT, Hirsch JG: Isolation of granules from eosinophil leukocytes and study of their enzyme
content. J Exp Med 118:277, 1963 66. Gleich GJ, Loegering DA, Kueppers F et al: Physiochemical and biological properties of the major basic protein from
guinea pig eosinophil granules. J Exp Med 140:313, 1974 67. Gleich GJ, Loegering DA, Mann KG et al: Comparative properties of the Charcto-Leyden crystal protein and the major
basic protein from human eosinophils. J Clin Invest 57:633, 1976 68. Frigas E, Loegering DA, Solley SO et al: Elevated levels of the eosinophil granule major basic protein in the sputum
of patients with bronchial asthma. Mayo Clin Proc 56: 345, 1981 69. Frigas E, Loegering DA, Gleich GJ: Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal
epithelium. Lab Invest 42:35, 1980 70. Fahy G, Easty DL, Collum L et al: Double masked efficacy and safety evaluation
of lodoxamide 0.1% ophthalmic solution versus opticrom 2%—a multicentre study. Ophthalmology Today p 341, 1988 71. Trocme SD, Kephart GM, Allansmith MR et al: Conjunctival deposition of eosinophil granule major basic protein in vernal
conjunctivitis and contact lens-associated giant papillary conjunctivitis. Am J Ophthalmol 108:57, 1989 72. Allansmith MR, Baird RS, Greiner JV: Vernal conjunctivitis and contact lens-associated giant papillary conjunctivitis
compared and contrasted. Am J Ophthalmol 87:544, 1979 73. Morgan G: The pathology of vernal conjunctivitis. Trans Ophthalmol Soc UK 91:467, 1971 74. Udell IJ, Gleich GJ, Allansmith MR et al: Eosinophil granule major basic protein and Charcot-Leyden crystal protein
in human tears. Am J Ophthalmol 92:824, 1981 75. Trocme SD, Kephart GM, Allansmith MR et al: Conjunctival deposition of eosinophil granule major basic protein in vernal
conjunctivitis and contact lens-associated giant papillary conjunctivitis. Am J Ophthalmol 108:57, 1989 76. Trocme SD, Kephart G, Bourne WM et al: Eosinophil major basic protein deposition in human corneal shield ulcers (abstr). Invest Ophthalmol Vis Sci 33 (suppl):94, 1992 77. Benveniste J, Chignard M, le Couedic JP et al: Biosynthesis of platelet-activating factor (PAF-acether). II. Involvement
of phospholipase A2 in the formation of PAF-acether and lyso-PAF-acether from rabbit platelets. Thromb Res 25:375, 1982 78. Braquet P, Toqui L, Shen TY et al: Perspectives in platelet-activating factor research. Pharmacol Rev 39(2):97, 1987 79. Benveniste J, Le Couedic JP, Polonsky J et al: Structural analysis of purified platelet-activating factor by lipases. Nature (London) 269:170, 1977 80. Benveniste J, Tence M, Varonne P et al: Semi-synthesis and proposed structure of platelet-activating factor (PAF): PAF-acether
an alkyl ether analog of lipophosphatidylcholine. CRC Acad Sci 289D:1037, 1979 81. Demopoulos CA, Pinckard RN, Hanahan DJ: Platelet-activating factor. Evidence for 1-alkyl-acetyl-sn-glyceryl-phosphorylcholine
as the active component (a new class of lipid chemical
mediators). J Biol Chem 254:9355, 1979 82. Tamura N, Agrawal D, Suliman FA et al: Effects of platelet-activating factor on the chemotaxis of normodense eosinophils
from normal subjects. Biochem Biophys Res Commun 142:638, 1986 83. Rubin RM, Samples JR, Rosenbaum JT: Prostaglandin-independent inhibition of ocular vascular permeability by
a platelet-activating factor antagonist. Arch Ophthalmol 106:1116, 1988 84. Braquet P, Toqui L, Shen TY et al: Perspectives in platelet-activating factor research. Pharmacol Rev 39(2):97, 1987 85. Braquet P, Vidal RF, Braquet M et al: Involvement of leukotrienes and PAF-acether in the increased microvascular
permeability of the rabbit retina. Agents Actions 15: 82, 1984 86. George MA, Smith LM, Berdy GJ et al: Platelet activating factor induced inflammation following topical ocular
challenge. Invest Ophthalmol Vis Sci 31 (ARVO Suppl):63, 1990 87. Klinman G, Butrus SI, Weston JH et al: Modulation of arachidonic acid metabolism in the rabbit conjunctiva (abstr). Invest Ophthalmol Vis Sci 24(suppl):200, 1983 88. Abelson MB, Butrus SI, Weston JH: Aspirin therapy in vernal conjunctivitis. Am J Ophthalmol 95:502, 1983 89. Wasserman M: Bronchopulmonary responses to prostaglandin F2 alpha, histamine, and acetylcholine in the dog. Eur J Pharmacol 32:146, 1975 90. Wasserman MA, DuCharme DW, Griffin RL et al: Bronchopulmonary and cardiovascular effects of prostaglandin D2 in the dog. Prostaglandins 13:255, 1977 91. Szczeklik A, Gryglewski RJ, Nizankowska E et al: Pulmonary and anti-platelet effects of intravenous and inhaled prostacyclin
in man. Prostaglandins 16:651, 1978 92. Cantarow WD, Cheung HT, Sundharadas G: Effects of prostaglandins on the spreading, adhesion, and migration of
mouse peritoneal macrophages. Prostaglandins 16:39, 1978 93. Mathe AA, Hedqvist P: Effect of prostaglandins F2 alpha and E2 on airway conductance in healthy subjects and asthmatic patients. Am Rev Respir Dis 111:313, 1975 94. Solomon LM, Juhlin L, Kirschenbaum MB: Prostaglandin on cutaneous vasculature. J Invest Dermatol 51:280, 1968 95. Crounkhorn P, Willis AL: Interaction between prostaglandins E and F given intradermally in the rat. Br J Pharmacol 41:507, 1971 96. Ferreira SH: Prostaglandins, aspirin-like drugs and analgesia. Nature New Biol 240:200, 1972 97. Leopold IH: Advances in ocular therapy: Noncorticosteroid anti-inflammatory agents. Am J Ophthalmol 78:759, 1974 98. Lewis RA, Holgate ST, Roberts LJ II et al: Preferential generation of prostaglandin
D2 by rat and human mast cells. In Becker, Simon AS, Austen KF (eds): Biochemistry
of the Acute Allergic Reactions (4th International Symposium), pp 239–254. New
York, AR Liss, 1981 99. Lewis RA, Soter NA, Diamond PT et al: Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol 129:1627, 1982 100. Roberts LJ II, Sweetman BJ, Lewis RA et al: Increased production of prostaglandin D2 in patients with systemic mastocytosis. N Engl J Med 303:1400, 1980 101. Roberts LJ II, Sweetman BJ, Lewis RA et al: Markedly increased synthesis of prostaglandin D2 in systemic mastocytosis. Trans Am Assoc Physicians 93:141, 1980 102. Abelson MB, Madiwale NA, Weston JH: The role of prostaglandin D2 in allergic ocular disease. In O'Connor GR, Chandler JW (eds): Third International
Symposium of the Immunology and Immunopathology of the Eye, pp 163–166. New
York, Masson & Co, 1985 103. Davies P, MacIntyre DE: Prostaglandins and inflammation. In Gallin G, Goldstein
I, Snyderman R (eds): Inflammation. Basic Principles and Clinical
Correlates. New York, Raven Press, 1992 104. Dhir SP, Garg SK, Sharma YR et al: Prostaglandins in human tears. Am J Ophthalmol 87:403, 1979 105. Bhattacherjee P: The role of arachidonate metabolism in ocular inflammation. Prog Clin Biol Res 312:211, 1989 106. Ellis EA, Oilz O, Roberts LJ et al: Coronary arterial smooth muscle contraction by a substance released from
platelets. Evidence that it is thromboxane A2. Science 193:1135, 1976 107. Hamberg M, Svensson J, Samuelsson B: Thromboxanes. A new group of biologically active compounds derived from
prostaglandin endoperoxides. Proc Natl Acad Sci USA 72:2994, 1975 108. Reiss J, Abelson MB, George MA, Wedner JH: Allergic conjunctivitis. In
Pepose J, Holland G, Wilhelmus K (eds): Ocular Infection and Immunity, p 347. Boston, Mosby, 1996 109. Goetzl EJ, Gorman RR: Chemotactic and chemokinetic stimulation of human eosinophil and neutrophil
polymorphonuclear leukocytes by 12-L-hydroxy,8, 10-heptadecatrienoic
acid (HHT). J Immunol 120:526, 1978 110. Blackwell GJ, Carnuccion R, DiRosa M et al: Macrocortin. A polypeptide causing the antiphospholipase effect of glucocorticoids. Nature 287:147, 1980 111. Hirata F, Schiffman E, Venkatasubramanian K et al: A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci USA 77:2533, 1980 112. Bisgard H, Ford-Hutchinson AW, Charleson S et al: Production of leukotrienes in human skin and conjunctival mucosa after
specific allergen challenge. Allergy 40:417, 1985 113. Ford-Hutchinson AW, Bray MA, Doig MV et al: Leukotriene B, a potent chemokinetic and aggregating substance released
form polymorphonuclear leukocytes. Nature 286:264, 1980 114. O'Flaherty JT, Showell JH, Becker EL et al: Neutrophil aggregation and degranulation. Effect of arachidonic acid. Am J Pathol 95:433, 1979 115. O'Flaherty JT, Showell HJ, Becker EL et al: Role of arachidonic acid derivatives in neutrophil aggregation. A hypothesis. Prostaglandins 17:915, 1979 116. Smith MJH: Biological activities of leukotriene B4 (isomer III). Adv Prostaglandin Thromboxane Leukotriene Res 9:283, 1982 117. Smith MJH, Ford-Hutchinson AW, Bray MA: Leukotriene B: A potential mediator of inflammation. J Pharm Pharmacol 32:517, 1980 118. Bhattacherjee P, Eakins KE, Hammond B: Chemotactic activity of arachidonic acid lipoxygenase products in the rabbit
eye. Br J Pharmacol 73:254P, 1981 119. Butrus SI, Corey EJ, Weston JH et al: The effect of leukotriene B4 in rabbit and guinea pig eyes (abstr). Invest Ophthalmol Vis Sci (Suppl):109, 1984 120. Bray MA, Ford-Hutchinson AW, Smith MJH: Leukotriene B4: An inflammatory mediator in vivo. Prostaglandins 22:213, 1981 121. Carr SC, Higgs GA, Salmon JA et al: The effects of arachidonate lipoxygenase products on leukocyte migration
in rabbit skin. Br J Pharmacol 73:253P, 1981 122. Atherton A, Born GVR: Quantitative investigation of the adhesiveness of circulating polymorphonuclear
leukocytes to blood vessel walls. J Physiol 222:447, 1972 123. Bray MA, Cunningham FM, Ford-Hutchinson AW et al: Leukotriene B4: A mediator of vascular permeability. Br J Pharmacol 72:483, 1981 124. Bisgard H, Ford-Hutchinson AW, Charleson S et al: Production of leukotrienes in human skin and conjunctival mucosa after
specific allergen challenge. Allergy 40:417, 1985 125. Spada CS, Woodward DF, Hawley SB et al: Leukotrienes cause eosinophil emigration into conjunctival tissue. Prostaglandins 31:795, 1986 126. Trocme SD, Gilbert CM, Allansmith MR et al: Characteristics of the cellular response of the rat conjunctiva to topically
applied leukotriene B4. Ophthalmic Res 21:297, 1989 127. Abelson MB: Lipoxygenase products in ocular inflammation (abstr). Invest Ophthalmol Vis Sci 25(suppl):42, 1984 128. Woodward DF, Ledgard SE: Comparison of leukotrienes as conjunctival microvascular permeability factors. Ophthalmic Res 17:318, 1985 129. Weiss JW, Drazen Jm, Coles N et al: Bronchoconstrictor effects of leukotriene C in humans. Science 216:196, 1982 130. Dahlen SE, Hedqvist P, Hammerstrom S et al: Leukotrienes are potent constrictors of human bronchi. Nature 288: 484, 1980 131. Hedqvist P, Dahlen SE, Gustafsson L et al: Biological profile of leukotrienes C4 and D4. Acta Physiol Scand 110: 331, 1980 132. Lewis RA, Austen KF, Drazen JM et al: Structure, function, and metabolism of leukotriene constituents of SRS-A. Adv Prostaglandin Thromboxane Leukotriene Res 9:137, 1982 133. Smedegard G, Hedqvist P, Dahlen SE et al: Leukotriene C4 affects pulmonary and cardiovascular dynamics in monkey. Nature 295:327, 1982 134. Hansson G, Bjorck T, Dahlen SE et al: Specific allergen induces contraction of bronchi and formation of leukotrienes
C4, D4, and E4 in human asthmatic lung. Adv Prostaglandin Thromboxane Leukotriene Res 12:153, 1983 135. Burke JA, Levi R, Corey EJ: Cardiovascular effects of pure synthetic leukotrienes C and D. Fed Proc 40:1015, 1981 136. Levi R, Burke JA, Corey JA: SRS-A, leukotrienes, and immediate hypersensitivity reactions of the heart. Adv Prostaglandin Thromboxane Leukotriene Res 9:215, 1982 137. Drazen JM, Austen KF, Lewis RA et al: Comparative airway and vascular activities or leukotrienes C1 and D in vivo and in vitro. Proc Natl Acad Sci USA 77:4354, 1980 138. Johnson HG, Chinn RA, Chow AW et al: Leukotriene C4 enhances mucus production from submucosal glands in canine trachea in vivo. Int J Immunopharmacol 5:391, 1983 139. Donnelly AL, Glass M, Minkwitz MC, Casale TB: The leukotriene D4 receptor antagonist, ICI 204,219, relieves symptoms of acute seasonal
allergic rhinitis. Am J Respir Crit Care Med 151(6):1734, 1995 140. Horak F, Toth J, Hirschwehr R et al: Effect of continuous allergen challenge on clinical symptoms and mediator
release in dustmite-allergic patients. Allergy 53(1):68, 1998 141. Bisgard H, Ford-Hutchinson AW, Charleson S et al: Production of leukotrienes in human skin and conjunctival mucosa after
specific allergen challenge. Allergy 40:417, 1985 142. Weston JH, Abelson MB: Leukotriene C4 in rabbit and human eyes (abstr). Invest Ophthalmol Vis Sci 26(suppl): 191, 1981 143. Udell IJ, Abelson MB: Chemical mediators of inflammation. Int Ophthalmol Clin 23(1):15, 1983 144. Vafeas C, Mieyal PA, Urbano F et al: Hypoxia stimulates the synthesis of cytochrome P450-derived inflammatory
eicosanoids in rabbit corneal epithelium. J Pharmacol Exp Ther 287(3):903, 1998 145. Lundgren JD, Shelhamer JH, Kalimer MA: The role of eicosanoids in respiratory mucus hypersecreation. Ann Allergy 55:55, 1985 146. Abelson MB: Lipoxygenase products in ocular inflammation (abstr). Invest Ophthalmol Vis Sci 25(suppl):42, 1984 147. Reiss J, Abelson MB, George MA, Wedner JH: Allergic conjunctivitis. In
Pepose J, Holland G, Wilhelmus K (eds): Ocular Infection and Immunity, p 347. Boston, Mosby, 1996 148. Cooper NR: Activation and regulation of the first complement component. Fed Proc 42:134, 1983 149. Pangburn MK, Morrison DC, Schreiber RD, Muller-Eberhard HJ: Activation of the alternative pathway. Recognition of surface structures
on activators by bound C3b. J Immunol 124:977, 1980 150. Hugli TE: The structural basis for anaphyloxin and chemotactic function of C3a, C4a, C5a. CRA Crit Rev Immunol 2:231, 1981 151. Raisman MB: Cellular products. In Gallin JI, Goldstein IM, Snyderman R (eds): Inflammation: Basic
Principles and Clinical Correlates. New York, Raven
Press, 1992 152. Foster S: Hypersensitivity reactions. In Albert, Jakobiec: Principles and
Practice of Ophthalmology. New York, WB Saunders, 1994 153. Willcox MD, Morris CA, Thakur A et al: Complement and complement regulatory proteins in human tears. Invest Ophthalmol Vis Sci 38(1):1, 1997 154. Mondino BJ, Ratajczak HV, Goldberg DB et al: Alternate and classical pathway components of complement in normal cornea. Arch Ophthalmol 98:346, 1980 155. Ballow M, Donshik PC, Mendelson L: Complement proteins C3 anaphylatoxin in the tears of patients with conjunctivitis. J Allergy Clin Immunol 76:473, 1985 156. Ihle JN, Pepersack L, Rebar L: Regulation of T cell differentiation. In vitro induction of 20 alpha-hydroxysteroid dehydrogenase in splenic lymphocytes
is mediated by a unique lymphokine. J Immunol 126:2184, 1981 156a. Magone MT, Whitcup SM, Chan CC et al: IL-12 is essential for the induction of late phase cellular infiltration
in a murine model of allergic conjunctivitis. FASEB 132 (Abstract): A339, 1999 157. Ihle JN, Rebar L, Keller J et al: Interleukin 3: Possible roles in the regulation of lymphocyte differentiation
and growth. Immunol Rev 98:101, 1981 158. Passalacqua G, Senna G, Dama A et al: The relationship between clinical efficacy of specific immunotherapy and
serum intercellular adhesion molecule-1 levels. J Invest Allerg Clin Immunol 8(2):123, 1998 159. Ciprandi G, Buscaglia S, Pesce GP et al: Allergic subjects express intercellular adhesion molecule-1 (ICAM-1 or
CD54) on epithelial cells of conjunctiva after antigen challenge. J Allergy Clin Immunol 91:783, 1993 160. Canonica GW, Ciprandi G, Passalacqua G et al: Molecular events in allergic inflammation: Experimental models and possible
modulation. Allergy 52(34 Suppl):25, 1997 161. Ciprandi G, Buscaglia S, Pesce G et al: Cetirizine reduces inflammatory cell recruitment and ICAM-1 (or CD54) expression
on conjunctival epithelium in both early- and late-phase reactions
after allergen-specific challenge. J Allergy Clin Immunol 95(2):612, 1995 162. Suzuma I, Mandai M, Suzuma K et al: Contribution of E-selectin to cellular infiltration during endotoxin-induced
uveitis. Invest Ophthalmol Vis Sci 39(9):1620, 1998 163. Abu el-Asrar AM, Geboes K, al-Kharashi S et al: Adhesion molecules in vernal keratoconjunctivitis. Br J Ophthalmol 81(12):1099, 1997 164. Thomas J, Kanangat S, Rouse BT: Herpes simplex virus replication-induced expression of chemokines and proinflammatory
cytokines in the eye: Implications in herpetic stromal
keratitis. J Interferon Cytokine Res 18(9):681, 1998 165. Tran MT, Tellaetxe-Isusi M, Elner V et al: Proinflammatory cytokines induce RANTES and MCP-1 synthesis in human corneal
keratocytes but not in corneal epithelial cells. Beta-chemokine
synthesis in corneal cells. Invest Ophthalmol Vis Sci 37(6):987, 1996 166. Fukagawa K, Saito H, Tsubota K et al: RANTES production in a conjunctival epithelial cell line. Cornea 16(5): 564, 1997 |