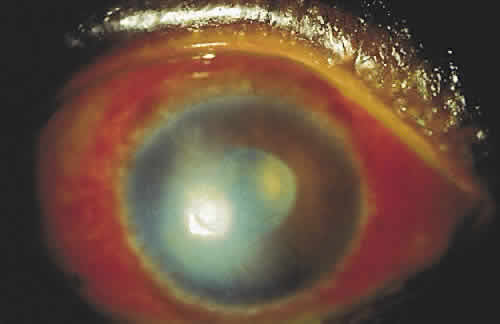
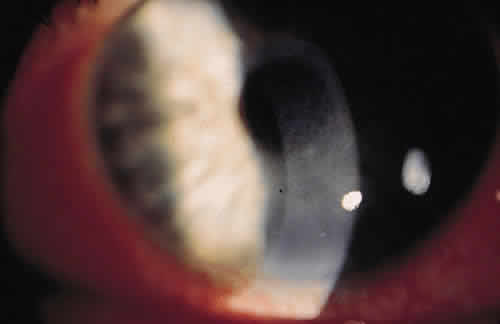
PHYSICAL BARRIERS
The physical barriers include the skin, tear contents, and conjunctiva. The initial interaction a potentially infectious organism may have is with the eyelashes. The eyelashes, or cilia, are sensitive to contact with external objects that initiate a blink reflex to mechanically protect the eye.7 If the bacterium reaches the skin, it does not survive long because of the direct inhibitory effect of the lactic acid and the fatty acids in sweat and sebaceous secretions and the low pH that these substances generate. Desquamation of the skin aids by shedding the adherent microorganisms.
If an organism is able to bypass the lids and lashes and has successfully reached the ocular surface, it interacts with various proteins, including lysozyme, lactoferrin, and lipocalin, which are present in the tear film and aid with nonimmunologic host defense. Lysozyme, which accounts for 40% of tear proteins, is a bacteriolytic enzyme that disrupts the peptido-glycan cell wall of susceptible organisms through a mechanism similar to that of penicillin.8 Although most gram-positive bacteria are affected by lysozyme, Staphylococcus aureus is an exception, because the lysozyme-susceptible site in the cell wall is blocked structurally from lysozyme attack. There is evidence that lysozyme levels decrease with age and with aqueous tear deficiency. This may contribute to the higher rates of outer eye infection in these groups.8 Lactoferrin makes up 25% of the proteins in tears. It enhances the function of natural killer cells, deprives bacteria such as staphylococci of iron, and may even have a direct effect on certain strains of bacteria.9 Lipocalin was recently recognized as playing a role in the nonimmunologic defense against microorganisms and viruses.10 Its function in the tear film is thought to be related to cysteine proteinase inhibition, which regulates protein metabolism and protects ocular tissue from proteolytic attack from bacteria and viruses.11
If the organism is able to make it through the lashes, past the skin and tear film, it reaches yet another barrier, the conjunctiva. Mucin secreted from goblet cells in the conjunctiva can inhibit the penetration of cells by viruses through competition with cell surface receptors for the viral neuraminidase.12 The conjunctiva is also able to increase blood flow in the presence of irritants that may represent potentially infectious agents. The blood contains products that participate in natural and humoral immunity.
BLOOD-BORNE NONSPECIFIC(NATURAL) IMMUNITY
Nonspecific immunity includes defense mechanisms present before a person is exposed to infectious microbes or other foreign macromolecules; it has no specificity and no memory.13 Included are phagocytic cells, eosinophils, natural killer cells, and various blood-borne molecules. Innate immunity is the phylogenetically oldest defense against microbes and gives them the opportunity to evolve strategies to resist innate immunity.14
For the factors of innate immunity to be available at the site of potential infection, the factors must be brought to the area of interest through the bloodstream. The acute inflammatory response to bacteria or tissue injury is characterized by capillary dilation and increased capillary permeability. This transfers to the conjunctival epithelium the neutrophils, which play a crucial role in the defense against pyogenic bacteria, such as pneumococci and streptococci. In addition to furthering the transfer of leukocytes, the increased capillary permeability brings about a massive transudation of bactericidal factors contained in the serum: C-reactive protein, defensins, properdin, and the complement system (Fig. 1). These factors aid in the adherence of bacteria to the polymorphonuclear leukocyte and ultimately in phagocytosis.2 Defensins, for example, are considered to be one of the earliest peptide effectors of innate immunity.15 They are released by neutrophils and are present in tears and in the ocular mucosa.16 Defensins have antimicrobial activity against gram-positive and gram-negative bacteria, fungi, and viruses and accelerate wound healing by their mitogenic effect on epithelial cells and fibroblasts.
|
Many types of innate immunity occur in the eye. One example is the immune response to lipopolysaccharide or teichoic acids, found on microbe cell walls but not on mammalian cells. Recognition of lipopolysaccharide by CD4-positive lymphocytes leads to the release of tumor necrosis factor (TNF) and IL-12, activating neutrophils and natural killer cells leading to macrophage activation and inflammation.
SPECIFIC IMMUNE DEFENSES
Specific immune responses are classified into two types: humoral immunity, which is mediated by antibodies produced by B lymphocytes, and cell-mediated immunity (CMI), which is mediated by T lymphocytes.2
In humoral immunity, immunocompetent cells recognize an antigen and then must go to a central processing site, the lymphoid tissue, to produce antibodies. There are interrelated central processing sites throughout the body called mucous membrane-associated lymphoid tissues (e.g., in the lungs, gastrointestinal tract, and lacrimal gland). They lead to antibody distribution in all sites despite antigenic insult at only one site. Recently, Knop and Knop17 described conjunctiva-associated lymphoid tissue that contained all components, including follicular spots, to be necessary for a complete immune response. Before the demonstration of conjunctiva-associated lymphoid tissue, the predominant teaching was that in human eyes, after antigen sensitization in the mucosa-associated lymphoid tissue (Peyer's patches of the intestine), immunoglobulin A (IgA) committed precursor B cells and sensitized T cells to reach the general circulation and eventually reside in the lacrimal gland.18 Beneath the surface epithelium of the lacrimal gland, the B cells undergo further antigen stimulation and become plasma cells that secrete IgA and IgG.
Immunoglobulins are present at a higher concentration in tears than in serum.7 However, the concentration of IgA in tears is directly proportional to the tear flow rate. A diminished tear flow rate leads to increased IgA concentration while a person sleeps. This higher concentration of IgA can protect the ocular surface from residual organisms by neutralizing toxins and viruses and inhibiting the adherence of bacteria to mucosal surfaces, thus limiting an organism's ability to colonize the eye.19
Cell-mediated immunity also plays an important role locally and systemically in the eye's defense against microorganisms. When a T lymphocyte becomes sensitized to a bacterial antigen, it releases a soluble factor (lymphokine) that can invest the macrophage with the power to destroy ingested organisms. The sensitized T lymphocyte also releases factors that can aggregate macrophages at the site of insult and hinder their departure from the site. Chemotactic factors for neutrophils, basophils, and eosinophils are also released.2 The leukocyte infiltrate may confine the pathogen and prevent its entrance into the interior of the eye, but may also contribute to the necrotizing inflammation of the corneal stroma seen in gram-negative bacteria and herpes simplex virus (HSV).20
In CMI, several cytokines and interleukins play a role. IL-2 and its receptor have been found to be important mediators of the ocular inflammatory response, and a recent study by Nussenblatt and coworkers used IL-2 humanized anti-Tac antibody (ZenapaxÞRM [daclizumab], Hoffman La Roche, Nutley, NJ) in patients with chronic noninfectious uveitis.21 The mechanism of action of cyclosporin is also related to the inhibition of the production and release of IL-2. IL-2 is necessary for the induction of cytotoxic T lymphocytes in response to alloantigenic challenge and plays a major role in cellular and humoral immune response. Chemokines are produced by nearly all human cells. IL-8 has been shown to selectively induce neutrophil and eosinophil chemotaxis and degranulation in response to HSV and adenovirus.22