Antigen-antibody reactions are based on molecular interactions between antigenic epitopes and specific polyclonal or monoclonal antibodies. Precipitin procedures such as double immunodiffusion and counterimmunoelectrophoresis have been supplanted by easier and more rapid techniques (Fig. 1). The development of hybridoma technology allowed monoclonal antibodies to be produced against many clinically relevant microbial antigens, including surface proteins and exotoxins. Several antigen detection tests are potentially applicable to ocular infections (Table 1) and are most useful when infectious agents are difficult to culture.1
TABLE 1. Immunologic Methods for Diagnosis of Ocular Chlamydial and Viral Infections
| Organism | Antigen Detection | Antibody Detection |
| Chlamydia trachomatis | DFA and EIA | Not generally useful |
| Adenovirus | IFA and EIA | Paired sera |
| Enterovirus | Not available | Not generally available |
| Herpes simplex virus | DFA, IFA, and EIA | Not useful except for primary infection |
| Varicella-zoster virus | DFA and EIA | Not useful |
| Cytomegalovirus | DFA | Not useful |
| Epstein-Barr virus | Not available | Determines recent vs remote exposure |
| Papillomavirus | Not available | Not available |
| Human immunodeficiency virus (HIV-1) | Available | Available |
DFA, direct immunofluorescence assay; EIA, enzyme immunoassay; IFA, indirect immunofluorescent assay.
IMMUNOFLUORESCENCE
Fluorochrome-conjugated antibodies allowed to react with appropriate microbial antigens can be detected by an immunofluorescent method. Fluorescein is the most common conjugate, resulting in a bright apple-green color by fluorescent microscopy. Unlike other immunodiagnostic methods, immunofluorescence and immunoperoxidase staining are microscopic techniques that allow the presence of inflammatory cells to be noted in the background.
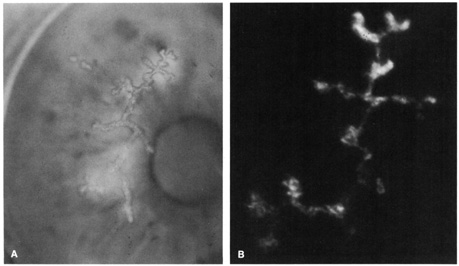
Direct immunofluorescence involves direct application of the fluoresceinated antibody to the specimen (Fig. 2). Indirect immunofluorescence entails mixing nonconjugated antibody with the specimen followed by application of a fluoresceinated antibody targeting the initial antibody (e.g., fluorescein-conjugated antihuman antibody). The indirect technique is usually more sensitive than the direct method. Distinguishing fluorescing apple-green particles from yellow, refractile debris relies on skilled observation.
Specimens are smeared onto a clean glass slide and fixed in cold methanol2 or acetone. An immunofluorescent test is useful for diagnosing herpes simplex virus epithelial keratitis,3–7,7a varicella-zoster virus epithelial keratitis,7 adenovirus conjunctivitis,8 and chlamydial conjunctivitis.9,10 Immunofluorescence and immunoperoxidase can also demonstrate several bacteria, fungi, and protozoa11,12,12a of ocular importance.
AGGLUTINATION REACTIONS
Particles coated with antibody can detect microbial antigens by producing agglutination. This immunochemical reaction causes visible clumping of the carrier material, whether erythrocytes, bacteria, synthetic beads, or phospholipid liposomes. Staphylococcus aureus is often used as a bacterial carrier in coagglutination reactions because its protein A naturally binds the Fc portion of most immunoglobulin subclasses, thereby leaving the immunoreactive end exposed. Latex and other inert materials (e.g., bentonite and collodion) are used in several commercial assays for evaluating common causes of meningitis and pharyngitis (e.g., Haemophilus influenzae, Streptococcus pneumoniae, group B streptococci, and Neisseria meningitidis) and can be applied to ocular infections.13 These assays detect soluble antigens as well as intact bacteria.
SOLID-PHASE IMMUNOASSAYS
Solid-phase assays are similar to agglutination reactions but use fixed polystyrene beads or wells that retain the microbial antigen. After the specimen is mixed with bound antibody, a tagged antibody is applied that further binds to the antigen, thus forming a sandwich of antigen between two antibodies. If the conjugate is a radioisotope (e.g., 32P or 125I), it is detected in a scintillation counter by radioimmunoassay. If the tag is an enzyme (such as horseradish peroxidase or alkaline phosphatase), the enzymatic substrate is added to yield a colored end product in an enzyme immunoassay, also called an enzyme-linked immunosorbent assay (ELISA). Chemiluminescent and other tags are under development. Several viral,4,14 chlamydial,9 bacterial, fungal, and parasitic antigens can be detected with commercially available enzyme immunoassays.
IMMUNODETECTION OF OCULAR INFECTIONS
Rapid diagnostic tests have high predictive value (Table 2) for adenovirus conjunctivitis,8,15,16 herpes simplex virus dendritic epithelial keratitis,4,7,12,17 and chlamydial conjunctivitis.9,18,19
TABLE 2. Performance of Immunodiagnostic TestsCompared to Culture for Ocular Infections
| Agent | Target Population | Test | Sensitivity | Specificity | Positive Predictive Value | References |
| Adenovirus | Acute follicular conjunctivitis | EIA | 51% | 99% | 97% | Nakagawa et al15, Wiley et al16 |
| DFA | 73% | 100% | 100% | Percivalle et al8 | ||
| Herpes simplex virus | Dendritic keratitis | EIA | 100% | 100% | - | Kowalski et al45 |
| DFA | 100% | 92% | 93% | Simon et al7 | ||
| IFA | 97% | 73% | 64% | Schwab et al46 | ||
| Chlamydia trachomatis | Adult chronic follicular conjunctivitis | EIA | 40% | 100% | - | Tantisira et al,18 Sheppard et al9 |
| DFA | 52% | 98% | 90% | Madhavan et al,47 Haller,48 Sheppard et al9 | ||
| Neonatal conjunctivitis | EIA | 97% | 99% | 97% | Hammerschlag et al49 | |
| Pediatric Trachoma | EIA | 73% | 93% | 92% | Schachter et al19 | |
| DFA | 78% | 100% | 100% | Schachter et al19 |
DFA, direct immunofluorescence assay; EIA, enzyme immunoassay; IFA, indirect immunofluorescence assay.