CONJUNCTIVA
The human conjunctiva is first exposed to microbes when an infant passes through the birth canal.13,14 Right after birth, the eye is exposed to environmental sources of bacteria such as air, contaminated material, and people. The flora obtained from the birth canal are predominantly aerobic and anaerobic bacteria (Table 1). The most common aerobic bacteria isolated immediately after birth from the conjunctiva are Staphylococcus aureus (coagulase-negative), diphtheroids (Corynebacterium species), Streptococcus species, Enterococcus species, and Escherichia coli. Propionibacterium acnes, Bifidobacterium, and Bacteroides species are isolated anaerobically from the newborn conjunctiva after vaginal delivery. Lactobacillus species, a microaerophilic bacteria, can also contaminate the conjunctiva from the vaginal tract. Other anaerobes and aerobes have been isolated less frequently (see Table 1). The conjunctiva is sterile in 20% to 87% of infants born vaginally.15 The conjunctiva cultured after cesarean section is sterile in 80% to 95% of infants.15,16 If cesarean section is delayed for more than 3 hours after the sac is ruptured, conjunctivas are sterile in 45% of newborns.
TABLE 41-1. Bacterial Flora of Normal Human Conjunctiva from Birth through
Various Age Groups
| Location | California | Korea | California | Korea | Washington | California | California | Georgia | Hawaii |
| Investigator | Brook, 197913 | Lee, 198916 | Brook, 197913 | Lee, 198916 | Weiss, 199260 | Singer, 198817 | Singer, 198817 | McNatt, 197861 | Brown, 197862 |
| Source of specimen | Newborns | Newborns | Newborns | Newborns | Children | Presurgical | Presurgical | Volunteers | Nursing home |
| Age | After birth | After birth | 48 hr | 48 hr | 4 mo to 12 yr | <17 yr | >17 yr | 22–60 yr | 26–99 yr |
| Number of eyes | 47 | 186 | 48 | 186 | 91 | 47 less than | 182 | 184 | 118 |
| Bacterial activity (no. isolates) | |||||||||
| No growth | 136 (73%) | - | 112 (60%) | 23 (25%) | 11 (23%) | 39 (21%) | 72 (39%) | 16 (14%) | |
| Coagulase-negative Staphylococcus | 10 (21%) | 28 (15%) | 17 (35%) | 40 (22%) | 9 (10%) | 14 (30%) | 108 (59%) | 43 (23%) | 57 (48%) |
| Staphylococcus aureus | 0 | 6 (3%) | 0 | 13 (7%) | 2 (2%) | 3 (6%) | 7 (4%) | 1 (0.5%) | 14 (12%) |
| Diphtheroids/Corynebacterium | 8 (17%) | - | 9 (18%) | - | 4 (4%) | 17 (36%) | 82 (45%) | 19 (10%) | 35 (30%) |
| Streptococcus viridans group | 9 (19%) | - | 9 (18%) | - | - | - | - | - | - |
| Streptococcus species | - | - | - | - | 1 (1%) | 7 (15%) | 4 (2%) | 4 (2%) | 1 (1%) |
| Enterococcus species | 1 (2%) | - | 1 (2%) | - | 1 (1%) | - | - | - | 5 (4%) |
| Micrococcus species | 2 (4%) | - | 8 (17%) | - | - | 0 < | 2 (1%) | 6 (3%) | - |
| Bacillus species | 5 (11%) | - | 1 (2%) | - | - | en>1 (2%) | - | - | - |
| Haemophilus species | 7 (15%) | - | 2 (4%) | - | - | 0 | 5 (3%) | - | - |
| Escherichia coli | 1 (2%) | 26 (14%) | 0 | 27 (15%) | - | - | - | - | 1 (1%) |
| Klebsiella species | - | - | - | 4 (2%) | - | - | - | - | 1 (1%) |
| Proteus species | - | - | - | - | - | - | 2 (1%) | - | 14 (12%) |
| Propionibacterium acnes | 6/27 (22%) | - | 10/18 (55%) | - | - | 6 (13%) | 55 (30%) | 72 (39%) | - |
| Bacteroides species | 7/27 (26%) | - | 0/18 | - | - | - | - | - | - |
| Peptostreptococcus species | 9/27 (33%) | - | 4/18 (22%) | - | - | 0 | 1 (0.5%) | 5 (3%) | - |
| Lactobacillus species | 3/47 (6%) | - | 1/48 (2%) | - | - | - | - | - | - |
| Eubacterium species | 1/27 (4%) | - | 2/18 (11%) | - | - | - | - | - | - |
| Clostridium species | 2/27 (7%) | - | 2/18 (11%) | - | - | - | - | - | - |
During the first several days of life, the infant's own cutaneous, respiratory tract, gastrointestinal flora, and the flora of the surrounding environment are important new sources of bacteria for the conjunctiva. After birth, before prophylaxis, there is a dramatic increase in the frequency of culture-positive conjunctivas, from as few as 13% of newborns to 98% of 3- to 5-day-old infants. Bacteria isolated include Staphylococcus species, Streptococcus species or Moraxella (Branhamella) catarrhalis.15
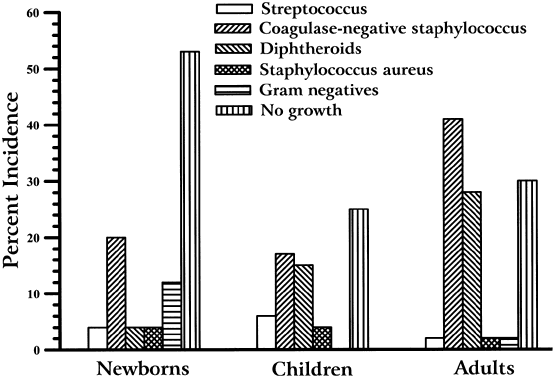
The literature (see Table 1) reports that from birth to old age, the conjunctiva is culture-negative for bacteria in 73% to 14% of patients. The frequency of isolating bacteria from the conjunctiva increases with age (Fig. 1). Coagulase-negative staphylococcus, diphtheroids (Corynebacterium species), P acnes, and S aureus are the most frequently isolated bacteria from the normal conjunctiva. Generally, the density of isolation is limited to fewer than 10 colonies on culture, compared with the confluent growth of infection. Streptococcus species are isolated more commonly from children than from adults. P acnes, diphtheroids, and gram-negative bacteria are isolated more frequent in adults.17,18 Less commonly, other gram-positive and gram-negative bacteria are isolated from the conjunctiva (Table 2). These isolates are probably transitory residents passed to the eye from other parts of the body and the environment by the adjacent skin or by the hands.
TABLE 41-2. Normal Flora of the Normal Human Conjunctiva Before Prophylaxis
and Surgery
| Location | Washington | Poland | Hungary | England | Saudi Arabia | Greece | Russia |
| Investigator | Boes, 199163 | Kecik, 199564 | Kovacs, 199465 | Bell/Walker, 86–8866,67 | Taylor, 198868 | Dereklis, 199469 | Karanadze, 198430 |
| Number of eyes | 100 | 2365 | 200 | 174 | 40 | 50 | 4927 |
| Bacterial activity (no. isolates) | |||||||
| No growth | 25 (25%) | 894 (38%) | 132 (66%) | 109 (63%) | 16 (40%) | 17 (34%) | 1166 (24%) |
| Coagulase-negative Staphylococcus | 59 (59%) | 1152 (49%) | 43 (22%) | 74 (43%) | 22 (55%) | 17 (34%) | 1372 (28%) |
| Staphylococcus aureus | 8 (8%) | 259 (11%) | 3 (2%) | 7 (4%) | 1 (3%) | 7 (14%) | 203 (4%) |
| Diphtheroids/Corynebacterium | 2 (2%) | 10 (0.4%) | 1 (0.5%) | 6 (3%) | 4 (10%) | 2 (4%) | 2138 (43%) |
| Streptococcus viridans group | 4 (4%) | - | 1 (0.5%) | - | 1 (3%) | 4 (8%) | - |
| Haemophilus species | 2 (2%) | - | - | 2 (1%) | - | - | - |
| Enterococcus species | 1 (1%) | - | 2 (1%) | - | - | 2 (4%) | 8 (0.1%) |
| Streptococcus species | 1 (1%) | 22 (1%) | - | 1 (0.6%) | 2 (5%) | - | 36 (0.7%) |
| Streptococcus pneumoniae | - | - | - | - | - | - | 80 (2%) |
| Micrococcus species | 0 | - | 14 (7%) | - | - | - | - |
| Bacillus species | - | - | - | 1 (0.6%) | - | - | 46 (1%) |
| Escherichia coli | 0 | 5 (0.2%) | 1 (0.5%) | - | - | - | 100 (2%) |
| Proteus species | 0 | 11 (0.4%) | 2 (1%) | 1 (0.6%) | 1 (3%) | 1 (2%) | 4 (0.1%) |
| Pseudomonas species | 0 | 7 (0.3%) | - | 1 (0.6%) | - | - | - |
| Citrobacter species | 0 | - | 3 (3%) | 1 (0.6%) | 1 (3%) | - | - |
| Klebsiella species | 1 (1%) | 5 (0.2%) | - | - | - | - | - |
| Propionibacterium acnes | 6 (6%) | - | - | - | - | - | - |
| Peptostreptococcus species | 3 (3%) | - | - | - | - | - | - |
Locather-Khorazo and Seegal14 reported that the bacterial flora did not differ in the eyes of healthy males and females, nor between the right and left eye. There were also no significant fluctuations during different seasons and no changes in the recovery rate of Staphylococcus species and diphtheroids on a yearly basis. The bacterial flora were the same for the conjunctiva and eyelid and frequently a mixture of Staphylococcus species and diphtheroids (Fig. 2). When comparing the bacterial flora of the conjunctiva with subjects from different countries, we found that the types of bacteria isolated are similar, although the frequency varies.
Table 2 details the bacterial flora isolated from human conjunctivas before surgery in selected parts of the world. The conjunctiva was 25% to 66% culture-negative. The conjunctivas were predominantly positive for coagulase-negative staphylococcus (22% to 59%); diphtheroids (Corynebacterium species, 2% to 43%); and S aureus (2% to 14%). Gram-negative bacteria on the conjunctiva were isolated more frequently in culture from eastern European countries. The isolation of gram-negative bacteria is generally limited to a few colonies on culture. Tomar and coworkers19 had reported a 6% recovery of Pseudomonas aeruginosa from the normal conjunctiva in India.
EYELID
The bacterial flora of the eyelid are similar to the bacterial flora of the skin. It is normal that a higher density of bacteria is isolated from the eyelid than from the conjunctiva. The normal human eyelid is always culture-positive for bacteria, and the bacterial types and quantities are random over time. Table 3 details the bacterial flora of the eyelid from several geographic locations. The types of bacteria are similar to those isolated from the conjunctiva. Coagulase-negative staphylococcus (575 to 100%) is the predominant bacterial isolate and generally exhibits heavy growth on culture. P acnes (71% to 74%), diphtheroids (8% to 45%), S aureus (12% to 16%), and Streptococcus viridans group (15%, children) are also frequently isolated with less density. A variety of gram-negative bacteria can also be isolated but less frequently and presenting with a few colonies.
TABLE 41-3. Flora of the Normal Human Eyelids
| Location | Washington | Florida | Oklahoma | Saudi Arabia | Ireland |
| Investigator | Weiss, 199260 | Groden, 199123 | Au, 199329 | Taylor, 198868 | Doyle, 199570 |
| Source of specimen | Children | Patients | Volunteers | Presurgical* | Presurgical |
| Number of eyes | 91 | 160 | 98 | 40 | 17 |
| Bacterial Activity (no. isolates) | |||||
| No growth | 0 | 0 | 0 | 0 | 0 |
| Coagulase-negative Staphylococcus | 52 (57%) | 140 (88%) | 88 (90%) | 38 (95%) | 17 (100%) |
| Staphylococcus aureus | 11 (12%) | 25 (16%) | -- | -- | 4 (10%) |
| Diphtheroids/Corynebacterium | 22 (24%) | 72 (45%) | 8 (8%) | 5 (13%) | 1 (6$) |
| Streptococcus viridans group | 14 (15%) | -- | -- | -- | -- |
| Enterococcus species | 3 (3%) | -- | -- | -- | -- |
| Streptococcus species | -- | -- | 1 (1%) | -- | 1 (3%) |
| Proteus species | -- | -- | 1 (1%) | -- | -- |
| Acinetobacter species | 4 (4%) | 7 (4%) | -- | -- | -- |
| Propionibacterium acnes | -- | 118 (74%) | -- | -- | 12 (71%) |
*Before prophylaxis.