TABLE 3. Definition of Microbial Keratitis and Aseptic
Keratitis With Distinguishing Clinical Characteristics
| Microbial Keratitis | Contact Lens–Associated Sterile Keratitis (CLASK) | |
| Definition | There is a high probability that replicating bacteria are the principle factors in the pathogenesis. Microbiologic investigations may not be positive. | There is a high probability that replicating bacteria are not involved in the pathogenesis. No laboratory investigations are available to confirm the diagnosis. |
| Clinical criteria | Lesions often central but can be in any location. | Peripheral lesions, occasionally central, often multiple. |
| Lesions > 1 mm in diameter. | Lesions usually < 1 mm may be > 1 mm, sometimes arcuate in the limbal zone | |
| Epithelial defect usually present but not mandatory. | Intact epithelium in early or mild lesions (contact lens–associated sterile infiltrative keratitis [CLSIK]). Ulcerated lesions with ulceration in severe or late lesions (contact lens–associated sterile ulcerative keratitis [CLSUK]). | |
| Pain, progressively deteriorates and may be severe. | Mild, non-progressive pain. | |
| Diffuse and/or severe progressive corneal suppuration. | Mild, non-progressive corneal infiltration. | |
| Iridocyclitis. | Minimal anterior chamber reaction. | |
| Asymptomatic lesions (contact lens–associated asymptomatic sterile keratitis [CLASK]). |
aIncludes the term presumed microbial keratitis.
bIncludes the terms peripheral corneal ulcers, sterile corneal infiltrates, contact lens–associated corneal ulcers, infiltrative keratitis, asymptomatic infiltrative keratitis
DISTINGUISHING INFECTIVE FROM OTHER CAUSES OF KERATITIS
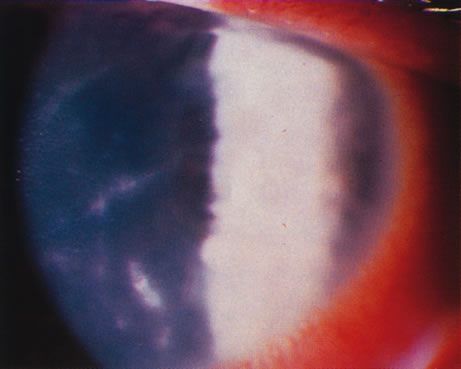
Differentiating MK from CLASK is important both because the management of the individual patient is quite different for the two disorders and because a satisfactory case definition is critical to the study of these different diseases. Current laboratory techniques cannot be reliably used to differentiate microbial from sterile keratitis. CLASK is a diagnosis of exclusion, both because there are no laboratory investigations to substantiate the different causes, and because microbiologic investigation in CLASK has a low sensitivity. It is well established that a negative corneal culture cannot be used to eliminate a microbial cause or, conversely, to validate a sterile cause. Large series of MK cases have documented a culture-positive rate of 50% in in-patient populations comprising the more severe cases,35 whereas a high proportion of CLASK cases are small lesions that may be expected to be culture positive less often. Negative culture results from microbial lesions may occur for several reasons: small volumes of infected material are available for culture, viable organisms present in deep tissues are less available to corneal sampling methods, and pretreatment with antibiotics reduces the viability of invading organisms. For these reasons, clinical criteria have to be used both for the diagnosis of individual patients and in studies designed to investigate these disorders. The size and position of the lesion and presence or absence of an epithelial defect31 are not diagnostic, although these criteria alone have often been used to attempt to differentiate the lesions. The clinical criteria that may be used to differentiate these lesions are summarized in Table 331,32 and illustrations of the lesions are shown in Figures 1, 2, 3, and 4. The use of clinical criteria has been supported by epidemiologic data showing that these definitions can distinguish between distinct disease processes.36
|
|
|
There is only sparse literature on keratitis associated with prosthetic CL use or the use of therapeutic CLs (TCLs). It is a reasonable assumption that those aspects of the epidemiology, pathogenesis and prevention of keratitis in prosthetic CL users that are determined by the materials, wearing pattern, and lens care systems will be similar to those in cosmetic CL userses. However, aspects determined by the ocular response to lens wear will be modified in unpredictable ways by the effects of prosthetic and therapeutic lenses on already abnormal ocular tissues. The following discussion refers principally to cosmetic CL wear although separate reference is made, where appropriate, to special considerations for prosthetic and therapeutic CL wear.
EPIDEMIOLOGY
Epidemiology is the study of the distribution and determinants of disease in a population. Epidemiologic studies have made a substantial contribution to our knowledge both of predisposing causes and of some of the factors to be considered in the pathogenesis of MK, particularly in CL wearers. Until the use of CLs became widespread as an alternative to spectacles, MK occurred in eyes with disrupted ocular defence mechanisms. The most important causes were corneal trauma and surgery, post-herpetic corneal disease, bullous keratopathy, corneal anaesthesia, corneal exposure, and the dry eye. CL wearers made up only a small proportion of patients with this disease.37–39 In the 1980s the number of CL users grew and the proportion of MK cases attributed to CL wear rose to over 50% in some centers, 3,5,40,41 making CL wear one of the major predisposing factors for MK in some populations. This problem has been of concern because, unlike other causes, it can be avoided and is potentially preventable.
Pathogenic Organisms in Therapeutic Contact Lens Users
TCL users are affected by a spectrum of organisms that differs from those affecting other contact lens users.5,41,42 Pseudomonas is a relatively rare cause of infection TCL users, whereas gram-positive organisms, particularly Streptococci, are common. Candida and other fungi,43 as well as unusual bacteria, are relatively frequent causes of keratitis in this group.44
Pathogenic Organisms in Cosmetic Contact Lens Users
In cosmetic CL users, bacteria and free-living amoebae are the principal causes of keratitis. It is an unfortunate accident of nature that Pseudomonas is one of the most virulent pathogens that can invade the cornea and Acanthamoeba among the most difficult to eliminate.
BACTERIA.
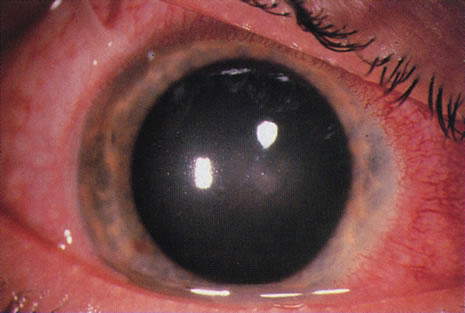
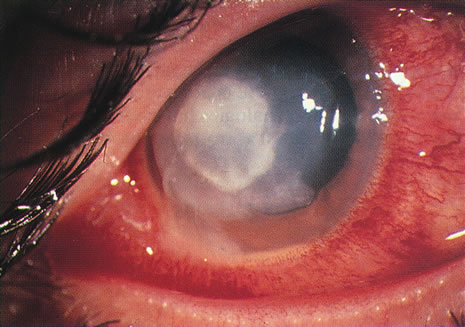
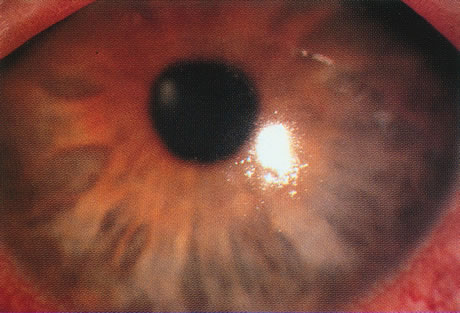
Bacterial infections predominate. However, polymicrobial infections are common and consist of any combination of bacterial types. Less often, bacterial keratitis may be combined with fungi, amebae, and herpesvirus. Descriptive studies and keratitis case series have shown that gram-negative bacterial infections predominate in cosmetic CL wear. Pseudomonas is the commonest organism (see Figs. 3 and 4) followed by gram-positive infections with Staphylococcus aureus and S epidermidis. Serratia is also frequently isolated with other gram-negative enterobacteria, including Escherichia coli.45
ACANTHAMOEBA.
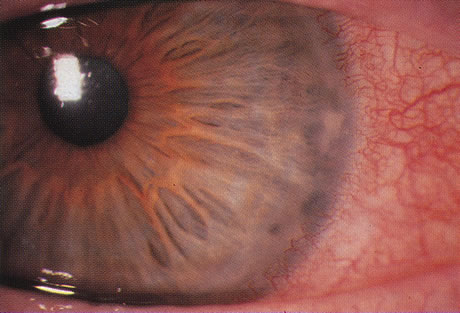
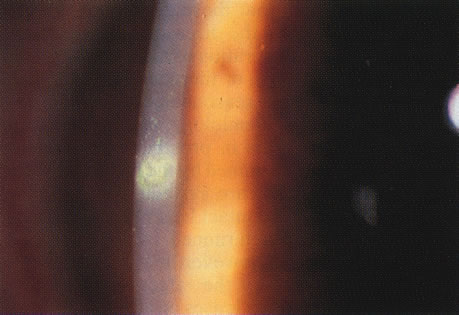
One of the most astounding developments in ocular microbiology in the last 15 years has been the emergence of Acanthamoeba as an important pathogen in CL users. Figures 5 and 6 show the early stages of Acanthamoeba keratitis. Before 1986 only 31 cases were reported to the Centers for Disease Control in the U.S., whereas in a 9-month period in 1986, 24 cases, 20 of which were CL-associated.46 The number of cases increased to about 200 by 1989; it was proposed that the increase partly resulted from the growth in popularity of CL wear,47 which is associated with Acanthamoeba infection in about 85% of cases.
|
The highest reported numbers of Acanthamoeba keratitis cases are from the UK, possibly as the result of the widespread use of domestic tank-stored water supplies in which the organism can proliferate.48 Acanthamoeba keratitis increased in the UK in the early 1990s, with one major center reporting 20 cases from 1984 to 1989, rising to 162 cases for the period 1990 to 1995.49,50 In Europe,the disease is possibly rarer, under-diagnosed,or under-reported.51,52
FUNGI.
Although fungi can contaminate the lens case and have the potential to invade soft lens materials, they have rarely been implicated as a cause of MK in CL users. It is likely that CL use has a minimal effect on the predisposition to fungal infection. Fusarium, Curvularia, and Paecilomyces have been reported.43,53 Fungal infection has also been reported in daily disposable and planned replacement SCL users.54,55
Table 4 summarizes some case series of keratitis and is divided into two periods; up to 1987,40,42,56–75 when Acanthamoeba was infrequently reported in CL users and when the range of CL types available was limited, and from 1988,54,55,76–79 when Acanthamoeba keratitis was reported in greater numbers at the same time that a wider range of lens types became available. Currently the proportion of CL keratitis cases resulting from Acanthamoeba keratitis varies from 5% to 25% of all culture-positive cases.76–79
TABLE 4. Summary of Case Series of Keratitis in Contact
Lens Users 1960–2001
| 1960–198740,42,56–75 | 1988–200154,55,76–79 | |
| Lens types | 199 | 47 |
| Reusable EW SCL | 5 | |
| Planned replacement EW SCL | 18 | |
| Disposable EW SCL | ||
| Reusable DW SCL | 99 | |
| Planned replacement DW SCL | 7 | |
| Disposable DW SCL | 1 | |
| Unknown type of SCL | 13 | 49 |
| Unknown type of DW SCL | 63 | |
| Any reusable or disposable SCL | 54 | |
| GP RCL | 34 | 52 |
| Therapeutic CL | 17 | 22 |
| Other | 3 | 32 |
| Organisms | ||
| Number (%) | Number (%) | |
| Gram-negative | 187 (40) | 98 (31) |
| Pseudomems aeruginosa | 141 (30) | 74 (23) |
| Serratia spp. | 13 | 10 |
| Other | 9 | |
| Gram-positive | 43 (9) | 54 (17) |
| Staphylococcus spp. | 35 (7) | 44 (14) |
| Other | 8 | |
| Mixed bacterial infections | 17 | |
| Unknown or unrecorded bacteria | 6 | 14 |
| Fungi | 8 | 5 |
| Acanthamoeba | 0 | 23 (7) |
| Total | 467 | 319 |
EW SCL, extended wear soft contact lens; DW SCL, dailywear soft contact lens; GP RCL, gas-permeable rigid contact lens.
HERPESVIRUS, RARE BACTERIAL CAUSES, AND MICROSPORIDIA.
Herpetic keratitis has not been associated with or apparently modified by CLs wear, although it is frequently misdiagnosed in CL users with amoebic keratitis, some of whom may have both conditions concurrently.80 Most of the rare bacterial causes of keratitis have been isolated from CL users, including Nocardia asteroides in EW SCL users,81 atypical mycobacteria in both RCL and SCLusers,82,83 and Microsporidia in an immunocompetent CL user.84 The importance of these uncommon associations is to be aware that CL users, like other patients, may be infected by a range of organisms; when the disease course is atypical, clinicians should be alert to the possibility of a less common cause. For example, the cases of fungal ring infiltrates in disposable CL wearers were initially mistaken for Acanthamoeba infection.55
Measuring the Incidence and Risks of Keratitis
Information about the incidence and risks of serious complication of CLs wear is needed both to ensure that lenses and lens care systems are safe and to give users and practitioners information that will allow them to make informed choices about the risks and benefits of different lens types and care systems. There is now heightened awareness of the potential problems associated with the use of different modalities of CL wear since the identification of the increased risks of extended wear in 1989 and 199085,86 and publication ofstudies showing increased, and largely avoidable, risks for Acanthamoeba keratitis in planned replacement DW SCL users.87
Information has come from a number of sources: national surveys, clinical trials, descriptive studies, population-based incidence studies, and case control studies. All these study designs have limitations that must be understood to interpret the data. National surveys have been limited by incomplete ascertainment of the cases and the size of the population at risk. Many small, prospective cohort studies of complications of different types of lens wear have usually been carried out as clinical trials. This design is generally inappropriate for the study of rare conditions because the size of the cohort may need to be too large to be practical. This has sometimes been taken to mean that the problem is too infrequent to be of concern. However, this is not the case when there is a very large population exposed or when the disease, like keratitis, may be severe. The limitations of this study design have often been overlooked when it has been used to assess the potential of CLs for keratitis and when data from clinical trials have provided little information about the risks for keratitis. For these reasons, descriptive studies in the form of the case reports and case series summarized in Table 4, rather than clinical trials, first identified the probability that there was an increased risk of keratitis associated with some CL types. Recently, the case control study design has been used to overcome some of the problems of clinical trials and to resolve the uncertainties arising from the results of descriptive studies in the assessment of risk for rare complications like keratitis. The case control study is ideally suited to investigating whether there are differences in risks among CL types, allowing comparison of new types of lens and lens-wearing regimens with those for which the level of risk is better established. The advantages and disadvantages of these different study designs have been reviewed.88 Case control studies have provided quantitative data on differences in risk for different lens types and other causes of keratitis. They have also been used to investigate, by multivariable analysis, the influence of additional factors that are associated with the use of different lens types and which might contribute to the risk of keratitis. The data derived from these different approaches to quantifying risk and incidence are describedin the following section.
Incidence and Risks of Keratitis in Groups with Medical Indications for Contact Lens wear
The incidence of keratitis in these groups has to be considered separately from that associated with cosmetic CL wear because of differences in the age of the users, characteristics of the lens, and differences in the ocular tissues which may affect the predisposition to keratitis.
Aphakic CLs users are usually elderly; the high plus power that is required in the lenses reduces the oxygen transmission; and corneal sensation and corneal metabolism are affected by cataract surgery. Because the number of patients at risk has been relatively small and the optical benefits of CL wear, as opposed to aphakic spectacle wear, is so great, keratitis in aphakic CL users has received relatively little attention. There have been a number of clinical trials evaluating aphakic CLs, most of which included a few cases of keratitis, unlike the similar studies carried out for the evaluation of cosmetic CLs. These studies showed an incidence of 0% to 6% during various follow-up periods.89–94 The numbers in these clinical trials were too small to establish a precise estimate for the incidence but these studies confirmed the clinical impression that it is relatively high compared to that for cosmetic CL use.
The most precise figures for the incidence of ulcerative keratitis in aphakic lens wear come from a population-based incidence study95 in which the overall annualized incidence for aphakic lens wear was estimated at 52:10,000 with a 7 times higher risk for extended wear compared to daily wear and 6 to 8 times greater risks for aphakic lens wear compared to cosmetic DW SCLs.
For TCL users the risk of infection is known to be high because of the abnormal ocular environment predisposing to infection.96–98 As outlined above, this group of CL users is affected by a different spectrum of organisms than other CL users, compared to whom TCL users have fewer gram-negative infections and a higher incidence of keratitis due to fungi, streptococci, and uncommon bacterial opportunists. Patients using these lenses for the management of bullous keratopathy and neurotrophic keratitis are frequently affected.44 In one study of TCLs for bullous keratopathy four of 30 patients developed keratitis.99
Incidence of Microbial Keratitis in Cosmetic Contact Lens Users
National surveys of CL-related disease have been carried out in both the UK and the U.S. In 1960 a postal survey of U.S. ophthalmologists documented six out of 49,950 eyes blinded from ulcerative keratitis associated with RCL wear in one year. Although no other cases were recorded, some with a better outcome did occur.56 In 1976, 13 cases of corneal infections were recorded in a postal survey of 54 consultant ophthalmologists in the UK over a 3-month period100 when the population of CL users could be only approximately estimated at 250,000. More recent and more precise estimates of the incidence of MK in CL users come from analysis of the pooled results of 48 consecutive pre-market approval studies (clinical trials) for the U.S. Food and Drug Administration, providing annualized incidence rates for keratitis of 6.8:10,000 for DW GPCL use, 5.2:10,000 for DW SCL use, and 18.2:10,000 for reusable EW SCL use.101 These studies were carefully carried out but were not comparative. They were conducted on carefully monitored volunteer users who had given informed consent, with individuals who failed to adhere to the follow-up schedules often excluded. For these reasons, such trials may not be representative of the population of CL users in the real post-marketing situation. Although the information provided by these studies is valuable, the individual studies were too small to give precise estimates of the incidence of keratitis in CL users. As a result, neither the licensing authorities nor CL practitioners were aware of the risks for keratitis associated with the use of EW SCLs.
The problems associated with the interpretation of these data have been successfully addressed in a population-based survey for estimating the incidence of ulcerative keratitis associated with CL wear in New England. This study estimated an annual incidence of ulcerative keratitis in the U.S. of 20.9:10,000 for EW SCL use compared to 4.1:10,000 for DW SCL use.102 The study did not have the power to identify differences between rigid and soft lens types.
A more recent well-designed national study from the Netherlands77 is remarkable in that it demonstrated the same incidence and risks for keratitis in GP, DW, and EW-SCLs that were demonstrated by the studies in the U.S. and the UK 10 years previously.4,29,102 This study is the only incidence study to date that included planned replacement SCL users; although the number of DW SCL users in this category was not reported, almost all the EW SCL users were on 1- to 2-week replacement schedules and were likely to have been using these lenses as disposable lenses. These studies are summarized in Table 5.
TABLE 5. Summary of Studies of Annualized Incidence of
Microbial Keratitis and Acanthamoeba Keratitis in Cosmetic Contact
Lens Wear
| Authors | Ulcerative Keratitis Rate Per 10,000 Subjects per yeara | ||
| GP RCL | DW SCL | EW SCL | |
| Poggio et al, 1989102 | Not estimated | 4.1:10,000 (2.9–5.2:10,000) | 20.9:10,000 (15.1–26.7:10,000) |
| MacRae et al, 1991101 | 6.8:10,000 (n = 3907) | 5.2:10,000 (n = 3591) | 18.2:10,000 (n = 1276) |
| Cheng et al, 199977 | 1.1:10,000 (0.6–1.7) | 3.5:10,000 (2.7–4.5) (included an unknown proportion of frequent replacement/disposable CL users) | 20:10,000 (10.3–35) (1–2 wk planned replacement/disposable CLs) |
| Incidence of Acanthamoeba Keratitis per 1,000,000 subjects per year | |||
| Location/year | Incidence per million population | Incidence per million CL users | |
| Radford et al, 1998103 | UK, 1992–1996 | 1.4 | 19.5 |
| Radford et al, 20022 | UK, 1997–1998 | 1.26 | 21.14 |
| UK, 1998–1999 | 1.13 | 17.53 (Wide regional variations in incidence, from 0–85 per million) | |
| Skarin et al, 1996106 | Sweden, 1991–1993 | 1.35 | |
| Schaumberg et al, 1998105 | U.S., 1985–1997 | 0.15–0.18 | 1.65–2.01 |
GP SCL, gas-permeable rigid contact lens; DW SCL, daily wear soft contact lens; EW SCL, extended wear soft contact lens.
a95% confidence limits where provided.
Incidence of Acanthamoeba Keratitis
The case definition for the studies described above was for MK, and almost all cases were proved or assumed to be due to bacteria; thus, they are effectively studies of bacterial keratitis in CL users. Because of the appearance of Acanthamoeba keratitis in the late 1980s and because of the severity of the disease, population-based studies have been carried out in the UK2,103 and the Netherlands.104 Other incidence estimates are available for the U.S.105 and Sweden106 (see Table 5). These studies demonstrate a much greater range of risks for CL users, with wide regional variations from 0 to 85 per million in the UK; the risks for non–CL users are low, and the estimates for the U.S. population are tenfold lower than for elsewhere. The high overall incidence in the UK and the regional variations have been related to the distribution of hard water,1 which results in limescale formation in domestic plumbing that provides an environment favorable to Acanthamoeba,107 combined with the widespread use of tank-stored water, which also favors the proliferation of the organism.48
Risk Factors for Microbial Keratitis in Cosmetic Contact Lens Users
The incidence studies previously described can be used to provide figures for the risk of keratitis using one CL type compared to another type. Another methodology for providing this data is the case control study, in which it is practical to use multivariable analysis to identify factors, other than the type of lens alone, which have contributed to the risk of infection. A number of case control studies have investigated the comparative risks of different CL types, and associated factors, both for microbial (principally bacterial) keratitis and for Acanthamoeba keratitis. Examination of the factors contributing to the development of keratitis has made a major contribution to our understanding of the pathogenesis of the disease. These are summarized in Table 6.4,29,36,77,87,108,109
TABLE 6. Summary of Studies of Risks of Corneal Infection
in Cosmetic Contact Lens Wear
| Author | Odds Ratiosa | |||
| Comparative risks for lens wear and other causes of MK | ||||
| Dart et al, 19914 | Ocular surface disease: | Trauma: | CL wear: | |
| 7.4x (2–25), PAR% (3.8%) | 13.9 (6–32), PAR% (22.4%) | 80.1x (389–167), PAR% (65.1%) | ||
| Contact lens type and odds ratios for MK | ||||
| GP RHCL: | PMMA RCL: | DW SCL: | EW SCL: | |
| Dart et al, 19914 | 1.0 (referent) | 1.3x (0.2–9.2) PAR% (0.8%) | 3.6x (0.9–13.9) PAR% (22.4%) | 20.8x (7.3–60) PAR% (44.4%) |
| Schein et al, 198929 | Not estimated | Not estimated | 1.0 (referent) | 5.15x (1.7–16) |
| Matthews et al, 1992109 | 1.0 (referent) | Not estimated | 1.1x (0.1–67.4) | Reusable: 4.1x (0.1–330) |
| Planned replacement: 13.3x (1.5–630) | ||||
| Radford et al, 1998108 | Not estimated | Not estimated | Reusable: 1.0 (referent) | Reusable: 1.0 (referent) |
| Planned replacement: 4.07 (1.7–4.1) | Planned replacement: 4.7 (1.52–14.87) | |||
| Cheng et al, 199977 | 1.0 (referent) | 1.0 (referent) | Includes reusable and planned replacement: 3.3x (1.9–6.1) | Disposable or planned replacement: 18.9x (10–35) |
| Other Statistically Significant Risk Factors for MK | ||||
| Radford et al, 1998108 | Occasional overnight lens use, | 3.95x (1.02–15.26) | ||
| poor lens case hygiene with chlorine systems; | 3.77x (1.42–9.98) | |||
| irregular disinfection. | 2.06x (1.03–4.14) | |||
| CL types and risks for Acanthamoeba keratitisb | ||||
| Radford et al, 199587 | Reusable DW SCL; 1.0 (referent) | Planned replacement DW SCL: 3.8 (1.01–14), p = 0.049 | ||
| Hygiene-Related Risk Factors for Acanthamoeba Keratitis | ||||
| Peroxide: | 1.0 (referent) | |||
| Chlorine with good compliance | 14x (3–76), p = 0.01 | |||
| Chlorine with poor compliance | 41x (7–232), p = 0.01 | |||
| No disinfection | 56x (10–302), p = 0.01 | |||
MK, microbial keratitis; CL, contact lens; GP RCL, gas-permeable rigid contact lens; PMMA RCL, polymethyl methacrylate rigid contact lens; DW SCL, daily wear soft contact lens, EW, extended wear soft contact lens.
aThe odds ratio approximates to an estimate of relative risk. Population attributable risk percentage (PAR%) is given where provided (95%confidence limits).
bRelative risks (95% confidence limits) p value.
REUSABLE SCLS.
One of the first case control studies showed that CLs were the major cause of MK (accounting for 65% of all new cases) and carried a risk for infection that was six times greater than that of corneal trauma alone.4 There are remarkable consistencies between the four best designed studies, spanning a period of 10 years.4,29,104,108 The two earliest studies were carried out in the U.S.29 and the UK,4 independently, to establish whether there was an increased risk associated with the use of EW SCLs which was an issue of national debate at that time. These both showed an increased risk of 5 times for extended wear of SCLs compared to daily wear. This finding was not confounded by the misuse of lens materials designed for daily wear (i.e.,being used overnight).
Multivariable analysis of case control study data has contributed to our understanding of the pathogenesis of keratitis in CL wear.4,29,36,108,121 This is as a result of identifying additional factors that are associated with the use of different lens types and which contribute to the risk of keratitis. Continuous periods of extended wear of more than 6 days were associated with a further increase in the risk of keratitis,4 which was incrementally related to the period of extended wear.29 Higher risks for keratitis are related to lower socioeconomic status, smoking, and being male — probably factors related to compliance with lens care advice. Surprisingly, no association between hygiene systems and compliance failures was shown in these studies, possibly because the studies had limited power to demonstrate relatively small effects. For keratitis with reusable DW SCL use, poor hygiene itself was shown to have a small but significant effect, and lens case cleaning was shown to be an important factor. However, levels of hygiene compliance were generally poor in both cases and controls.29 Multivariable analysis of more detailed questionnaires in UK studies allowed the effects of hygiene system and compliance to be more fully evaluated for reusable and planned replacement DW SCL patients36,108; these studies showed that both irregular and omitted disinfection, the use of chlorine systems (since withdrawn in the UK), and heat disinfection , now rarely used, all lead to a significantly higher risk of keratitis. These findings are summarized in Table 6. Although poor hygiene compliance increases the risk of keratitis for both planned replacement and reusable DW SCL users, it has been observed that a proportion of keratitis cases have good hygiene and uncontaminated lens care materials,3,122 suggesting that lens hygiene is not the only determinant of keratitis in this group.109 This is not surprising when the high contamination rate of lens storage cases of asymptomatic CL users, associated with current hygiene systems, is considered.123
FACTORS NOT CONFIRMED TO BE RELATED TO THE RISKS OF KERATITIS.
For both daily and extended wear of reusable and planned replacement CLs, many other factors that had been anticipated to affect the risk of developing keratitis have not been proved to do so, that is, any effect must be small, if present. These included factors that might be expected to have a relationship to decreased hygiene compliance, the age of the user, the number of years of lens wear and the period since the last follow-up visit. In addition, there was no effect of lens age on keratitis. This is at variance with what had been predicted from laboratory studies (described later) that show increased bacterial adherence to deposits on lenses; this was expected to result in an increased risk of keratitis with lens age. However, these epidemiologic findings suggest that increased lens contamination due to deposits does not affect the risks of keratitis, and that other factors must be more important.
PLANNED REPLACEMENT DW SCLs, DAILY DISPOSABLE DW SCLs, DISPOSABLE EW SCLs, AND KERATITIS.
In 1989, planned replacement soft lenses were introduced for daily wear and as disposable lenses for extended wear; daily disposable lenses were introduced shortly afterward. Planned replacement and disposability were concepts that were introduced to reduce the complications of CL wear, including infection. These lenses have gained an increasing market share because of a reduction in the minor complications of lens wear,110 in particular that of CL-related papillary conjunctivitis.107,113 There was some skepticism about the rationale for their use, both for extended wear and for daily wear, at a time when planned replacement CLs were still exposed to the contaminated environment of the CL case.86,114 Soon after their introduction, case reports of both bacterial115–118 and Acanthamoeba119 keratitis were published. These were followed by two case control studies, without multivariable analysis, that showed that planned replacement DW SCLs and disposable EW SCLs were probably no different, or worse, than reusable lenses with regard to the risk of keratitis.109,120 The raw results of these two studies may have been confounded by other factors that were not included in the analysis, such as the possibility that hygiene compliance levels might have been different in the users of planned replacement and reusable SCLs.
These questions were later resolved by two subsequent studies that showed that there was a slightly increased risk of MK for the users of planned replacement DW SCLs and disposable EW SCLs108 and that for EW disposable and planned replacement SCLs the risks were unchanged in the 10-year period since the previous studies—that is, 5 times higher compared to DW SCLs.77 These studies leave no doubt that the major risk factor for MK is overnight wear and that, for reusable and planned replacement hydrogel lenses, this cannot be modified by the elimination of the CL case and hygiene systems. Daily disposable SCL use may have reduced, but not eliminated, the risk of MK. Although there is no incidence or case control study including this lens type, there is a widely held perception among clinicians that fewer cases of keratitis than expected are currently being seen and that this correlates with the increasing use of daily disposable CLs. A case series of keratitis from one center reporting both the number of cases and associated lens types has provided some support for this view.78
ACANTHAMOEBA KERATITIS.
The effect of risk factors, other than lens type, has been shown to be much more important in the development of Acanthamoeba keratitis than for bacterial keratitis in CL wearers (for whom the effect of overnight wear is the overriding risk factor with relatively small measurable effects from hygiene practicesee the preceding discussion). For these reasons, the epidemiology of Acanthamoeba keratitis demands separate consideration from that of bacterial keratitis with the implication that, unlike bacterial keratitis, it is an almost entirely avoidable disease in CL users.
An early case control study of SCL-related amoebic keratitis showed that these infections were associated with the use of home-made saline solution, as opposed to proprietary solutions, and with the habit of swimming while wearing CLs.124 Until recently no differences in risk had been identified in association with different types of CLs.124–126 However, soon after the introduction of planned replacement CLs in the UK, the number of cases of Acanthamoeba keratitis increased. A large case series of Acanthamoeba keratitis, including all cases identified between 1984 and 1992, showed that 28/64 consecutive CL users with Acanthamoeba keratitis were using planned replacement lens systems.49 Planned replacement lenses were not introduced into the UK until 1989, and this proportion was much higher than expected from the market penetrance of the lenses at that time. This finding precipitated a case control study87 to establish the factors responsible.
The raw data from the case control study, before the multivariable analysis was done, showed a greatly increased risk associated with the use of planned replacement DW SCLs. However, the multivariable analysis showed that this finding was principally the result of a much poorer standard of lens hygiene in planned replacement SCL users than in reusable SCL users (see Table 6). Omission of hygiene practices increased the risk of keratitis by 56×. In addition, this frequent replacement lens system had been marketed with a chlorine release hygiene system, which is ineffective against Acanthamoeba. Use of this chlorine disinfection system increased the risk for Acanthamoeba keratitis between 14 times and 41 times, depending on how carefully it had been used. The difference in lens hygiene practice between the users of planned replacement DW SCLs and reusable DW SCLs could be attributed to the way in which the planned replacement lenses had been marketed as a “low care” system. Following the publication of this paper, the substantial publicity may have contributed to improved hygiene compliance and reduced incidence of Acanthamoeba keratitis in the UK.127 Subsequent incidence studies confirmed that most CL users who had developed Acanthamoeba keratitis had been exposed to one of the known risk factors for the disease, suggesting that, with due care, the disease is avoidable. These risk factors include swimming while wearing CLs, irregular or absent disinfection, use of both chlorine and one-step peroxide disinfection systems, use of non-sterile water and/or home-made saline, and poor lens case hygiene.2,103
RIGID GAS-PERMEABLE CONTACT LENSES.
The RGP CL has been of less interest because it has been perceived as a safe lens wear modality; it is currently in use by approximately 10% to 15% of all CL users in Europe and the U.S. Case control studies that have included this lens type have shown that the risk of MK is about 3 times less with RGP CLs than with daily wear SCLs4,77,108 and that only 1:10,000 RGP CL users will develop MK.
SILICONE HYDROGEL EXTENDED WEAR CONTACT LENSES.
This new modality, introduced in 1999, may be associated with reduced risks of keratitis attributed to the great improvement in oxygen transmission to the cornea with this lens. Pre-market studies and post-marketing surveillance data have been very encouraging,128 but further data is needed to confirm this before encouraging increased use of extended wear contact lenses.9
The Impact of Cosmetic Contact Lens–Related Keratitis on Populations
The data summarized in Tables 5 and 6 can be used to provide approximations of annualised incidence rates of CL-related keratitis for national populations. Table 7 shows this for the U.S. population in the year 2000. These figures demonstrate the effect on the numbers of keratitis cases that can result from relatively small changes in the proportions of different types of CLs worn. For example, if the use of disposable hydrogel EW SCLs were to be prohibited,more than 9,000 cases of keratitis could be avoided. These estimates demonstrate the importance of relatively small individual risks to the burden of disease when a large population is exposed to them.
Table 7. Estimates of the Effect of the Use of Different Lens Types on
the Annualized Incidence of Lens-Related Microbial Keratitis in
the U.S. in 2000a
| CL Type | Incidence estimatesb | 34 million users | Estimate of keratitis cases |
| Disposable hydrogel EW SCL | 1:500 | 17% (5.7 million) | 11,560c |
| Planned replacement (not daily disposable) DW SCL | 1:2500 | 44% (11.56 million) | 4,624 |
| Daily disposable DW SCL | Unknown | 44% (11.56 million) | Unknown |
| GP RCL | 1:10,000 | 15% (5.1 million) | 510 |
aData on number of wearers and proportions of different lens types in use are from the Contact Lens Council, 8201 Corporate Drive, Suite 850, Landover, MD 20785.
bFrom data in Tables 5 and 6.
cIf these individuals used their lenses as planned replacement DWSCLs, the number of keratitis cases in this group would be reduced to 2280, a reduction of 9280.
CL, contact lens; EW, extend wear soft contact lens; DW SCL, daily wear soft contact lens; GP RCL, gas-permeable rigid contact lens.
PATHOGENESIS OF MICROBIAL KERATITIS IN CONTACT LENS USERS
The epidemiological studies described previously identified several factors that contribute to the pathogenesis of keratitis in CL users: overnight wear, soft lenses, poor hygiene compliance, and certain hygiene systems. However, epidemiologic studies themselves cannot determine the pathogenesis of disease. This section integrates the epidemiologic data with the clinical and laboratory investigations that have led to our current understanding of the pathogenesis of corneal invasion by bacteria and Acanthamoeba in CL users; the pathogenesis of inflammation and corneal destruction by these organisms is described in other chapters.
Until recently, theories of the pathogenesis of MK in CL users were founded on two principal proposals. First, CL wear, particularly the use of EW SCLs, has extensive effects on the ocular surface129 that might be expected to compromise its resistance to microbial invasion.71,74,130 Second, some of these eyes are exposed to large numbers of bacteria that contaminate a high proportion of CL cases.131,132 The combination of increased susceptibility to infection and increased exposure therefore results in an increased risk of infection. Poor understanding of the pathogenesis of infection in CL users and of the mechanisms underlying these two proposals contributed to both the exacerbation and the perpetuation of the problem of MK in CL wear. An example of a solution is the introduction of extended wear, both reusable and disposable, as a means of reducing exposure to contaminated CL solutions.
In addition, these proposals do not address the questions of why Pseudomonas and Acanthamoeba are more common than other organisms in CL users with keratitis, why there is no link between CL case contamination and keratitis in all patients with keratitis,3,133 and why RCL users are probably at a lower risk for keratitis than SCL users. Some of these observations have now been investigated, contributing to the development of a theory for the pathogenesis of MK in CL users. The following contributory factors must be addressed for both bacterial and amoebic keratitis:
Source of the organisms
Role of microbial adherence to the CL
Colonization of the CL and CL case by bacteria
Role of the CL in reducing corneal resistance to microbial invasion
BACTERIAL KERATITIS
Source of Bacteria
All of the organisms that commonly cause keratitis in CL users may be isolated from the ocular surface of normal individuals. These include S. aureus, Pseudomonas aeruginosa, and other coliform bacteria.134 These organisms derive from several sources. S. aureus is a widespread human pathogen that is frequently commensal in the nose. P. aeruginosa is a ubiquitous organism in our environment, common in soil, on vegetation, in water, and in the human gut and upper respiratory tract.135 It has also been found to contaminate cosmetics as well as CL care solutions.136 Studies of the conjunctival flora in CL users have shown no qualitative change in the short term, and the numbers of bacteria may be reduced compared to controls (see section on conjunctivitis). However, one study has related conjunctival cultures to contamination by gram-negative flora from contaminated CL cases.22 These have since been shown to be an important additional source of pathogens.136–139 The importance of contamination of CL cases in the pathogenesis of bacterial keratitis in CL users has been known for many years, and the expected link between CL case contamination and keratitis has been confirmed in some studies,131 although an association is not always present.133 CL case contamination probably results from a combination of poor hygiene pracitces and the failure of current disinfection systems in use. The role of the CL case may be to amplify the concentration of the environmental bacteria contaminating the case, thus providing a large inoculum of microorganisms presented to the eye by the CL. The discovery of the potential importance of the bacterial glycocalyx (described later), provides a theoretical mechanism whereby small numbers of bacteria from these environmental sources might be able to amplify in large numbers, both in the CL case and on the CL surface itself.137
The Role of Bacterial Adherence
BACTERIAL ADHERENCE MECHANISMS.
If the contact lens is to act as a vector for bacteria, these must at first adhere to the lens surface. This requires that the bacteria reach the surface, either by fluid or airborne mechanisms. Attraction or repulsion occurs due to the sum of the Van der Waals forces and the electrostatic forces. This is mainly a function of the distance of the bacteria from the surface. These interactions of total Gibbs energies are described by the Derjaguin-Landau and Verwey-Overbeek (DLVO) theory.140 The net result is usually repulsion, but temporary adhesion may occur. This can be broken by mechanical forces or bacterial mobility.141 For a surface bathed in fluid, such as a contact lens, these forces become dependent on the concentration of solutes in the solution. Repulsive electrostatic forces are less effective as ionic strength is increased. They are also influenced by the size of the bacteria and the surface hydrophobicity. Hydrophobic bonding has been shown to be important on many acellular surfaces, outweighing the effect of surface charge, and the degree of adherence may be related to it.142–144 Specific lectin-like bonding, probably mediated by adhesins on bacterial pili, is probably more important in mediating adherence at mucosal surfaces than at acellular surfaces,144 although corneal infection may be caused by non-piliated strains.145 However, lectin-like bonding may be an important factor on lens surfaces covered in ocular deposits, including mucin, which contains sialic acid to which specific Pseudomonas receptors have been demonstrated.146
BACTERIAL ADHERENCE TO CONTACT LENSES.
Numerous studies have been carried out on bacterial adherence to contact lenses to explore the potential for adherent bacteria to cause keratitis. These studies have shown several inconsistencies. Both P. aeruginosa and S. aureus adhere to new and worn SCL and unworn polymethyl methacrylate (PMMA) RCL surfaces, with S. aureus having a much greater affinity for PMMA than Pseudomonas or to soft lenses.147 The greater adherence of S. aureus to PMMA than to the hydrogel SCL materials is predicted by the hydrophobic model of adherence, because PMMA has a surface hydrophobicity in the range associated with maximum bacterial adherence,142 and PMMA is more hydrophobic than are hydrogels.148 Subsequent studies, principally on Pseudomonas, have confirmed that Pseudomonas adheres in large numbers to both new149 and worn lens surfaces. Greater numbers of bacteria may adhere to worn surfaces150 and to focal deposits on SCLs, to which Pseudomonas adheres more avidly than S. aureus.151 Although the effect of ocular deposits in most studies has been to increase lens adherence,151,152 these experiments may represent an oversimplification of the in vivo situation and may have masked interstrain differences and interhost variations in the effect of ocular deposits on adherence. It has been shown that interspecies and interstrain variations in bacterial adherence to lenses occur.153 Surface deposits may or may not enhance the adherence of Pseudomonas.154,155 Inhibition of Pseudomonas adherence in worn lenses has been shown in a rabbit model.156 The hydrophobicity of the CL surface has been shown to dictate composition of the adsorbed tear film, and this then modifies bacterial adhesion to the CL surface.157. Therefore, although bacterial adherence to the lens surface is probably an important factor in the pathogenesis of keratitis, the clinical relevance of enhanced adherence to lens surfaces, when this occurs, has not been not proved. Laboratory findings implying enhanced adherence due to surface deposits do not correlate with case control study findings (already described), which failed to establish an association between lens ageing and keratitis,4,108 or with the findings of the case control studies showing increased risks for keratitis with planned replacement lens use.108
Although these studies have shown that the lens may act as a vector for the delivery of organisms to the eye from a contaminated lens case, it is also probable that the bacteria are able to colonize (replicate in microcolonies on) the surface of the lens. This involves the elaboration of a glycolcalyx by the bacteria and the maturing of this into a biofilm. As a strategy for bacterial survival on a surface, bacterial adherence is important in retaining viable bacteria long enough on the lens surface for a glycocalyx, and later a biofilm, to form.158 The development of these structures results in conversion of reversibly adhering bacteria, that are simply passengers on the lens surface, into replicating bacteria colonizing the lens surface in strongly attached populations. Subsequent to this, adherence of individual bacteria to the surface is likely to be of little significance.
Colonization of the Lens and Lens Case by Bacteria
BACTERIAL GLYCOCALYX AND BIOFILM IN THE PATHOGENESIS OF INFECTION.
In appropriate environmental conditions, most bacteria will secrete a glycocalyx that serves to bind microcolonies of bacteria together. The glycocalyx is a polysaccharide-containing structure produced by the bacteria and lying outside the peptidoglycan and outer membrane of gram-positive and gram-negative organisms.159 This structure is difficult to stabilize for visualization by electron microscopy and is lost when using routine fixation techniques with glutaraldehyde without using specific techniques for stabilization of the structure, such as the use of ruthenium red. Proliferation and organization of bacterial glycocalyx leads to the development of a more complex biofilm that results in irreversible adhesion of bacteria to the surface.158 A biofilm can be defined as a functional consortium of microorganisms organized within an extensive exopolymer matrix.160 It becomes a microcosm within which bacteria replicate, existing either as clusters of organisms or as isolated units. Nutrients may be trapped, bound, and recycled within the matrix but, in general, the bacteria are relatively deprived of nutrition and divide 5 to15 times slower than under planktonic (unattached in suspension) conditions.160a–162 This mechanism for bacterial colonization has been shown to be important in natural ecosystems, as well as on mucosal surfaces,163 in osteomyelitis,164 and on the surface of prostheses.165 Bacterial microcolonies in the glycocalyx are in the “sessile” state, residing within the biofilm where antibody and bacteriophage access to the bacteria is limited. Consequently, bacteria within a biofilm are better able to survive against white blood cells, bacteriophages, amoebae, antibiotics, biocides, surfactants, and mechanical trauma.166–172 However, bacterial antigens may still present at the biofilm surface. The resultant antibody-antigen interaction is often unable to effect bacterial lysis because the bacteria are protected deep in the biofilm.173 These biofilm-enclosed organisms may become mobile, planktonic, or “swarmer” cells when they are free to invade surrounding tissues. In this latter state they do not have the protection afforded by the glycocalyx from phagocytosis, bacteriophages,174 and antibiotics. In addition, these planktonic cells are genotypically identical but phenotypically different from the sessile cells and are particularly sensitive to antibiotics compared to the cells that remain within the biofilm,175–177 as well as to biocides.170 Clinically this difference in susceptibility may result in eradication of the planktonic cells by biocides or antibiotics but persistence of viable bacteria within the biofilm from where further shedding can occur. In vitro data show that the levels of antibiotic must be 20 to 1000 times greater in a biofilm to achieve adequate growth inhibition compared to the same bacteria when they have been liberated into a planktonic state.171,178,179 Similar data exists for biocides.159 This resistance of biofilm to antibiotics and biocidal agents is complex and not completely understood. Three major mechanisms are involved.
First, antibiotic or biocide access to the bacteria may be limited. Theoretically this could be due to impaired diffusion or to binding and/or inactivation of the antibiotic. However, the diffusion coefficients of antibiotics are similar to those of aqueous solutions,180,181 and the actual penetration of antibiotics is unimpaired by the biofilm.182 Selective binding of antibiotics by exopolymers does occur and is significant for ciprofloxacin and iodine but not tobramycin.176,181,183 Bacteria may liberate enzymes such as beta-lactamase that inactivate antibiotics. These enzymes are concentrated within the biofilm, and this causes a decreasing concentration of antibiotic adjacent to the bacteria.184 These last two factors are major contributors to the relative antibiotic resistance.
Second, nutrient availability within the biofilm may reduce the bacterial growth rate. This may cause the cells to adopt a different phenotype to that of planktonic growth. Both of these factors may alter the antibiotic sensitivity.185,186
Third, attachment to a surface may cause inducement and derepression of genes associated with a sessile existence, which may coincidentally alter antimicrobial sensitivity. Interference with this process could prevent bacterial adherence or prevent further production of the biofilm matrix.187
The role of bacterial glycocalyx and biofilm in contact lens–related keratitis.
It is probable that biofilm-enclosed bacterial colonization of surfaces is the rule rather than the exception and that the planktonic or free-living mode of existence that has been studied in the laboratory test tube is an artificial environment for most bacteria.
The kinetics of the development of glycolcalyx suggest that glycocalyx formation can occur rapidly enough for it to be the form in which bacteria are transported to the eye on a CL contaminated in its case. Therefore it is probable that, in different circumstances, the lens promotes keratitis by acting both as a vehicle for mature bacteria and as a surface for bacterial growth and reduplication. In addition, it has recently been suggested that the lens may act as a reservoir for organisms introduced into the conjunctiva from environmental sources other than the CL case, such as contaminated fingers or water. In these circumstances, adherence to the lens may result in a relative increase in the retention time of bacteria at the ocular surface, from a few hours to several hours, as determined by the life of the bacteria. It will not result in an increase in their numbers, or in more prolonged exposure, unless the adherence results in colonization of the lens surface. Recent interest has focused on the formation of lens surface biofilms that would allow this to occur.188–190
If a biofilm is developed by bacteria on the CL surface, this will enable the bacteria to colonize the surface and persist in the eye for prolonged periods. That organisms can colonize lens surfaces without causing keratitis has been shown in a rabbit model in which the numbers of organisms colonizing the lens in biofilm increased in the week following inoculation with Pseudomonas. Although the kinetics of biofilm formation in vivo has not been established, biofilm was well developed by 3 days in this animal model.189
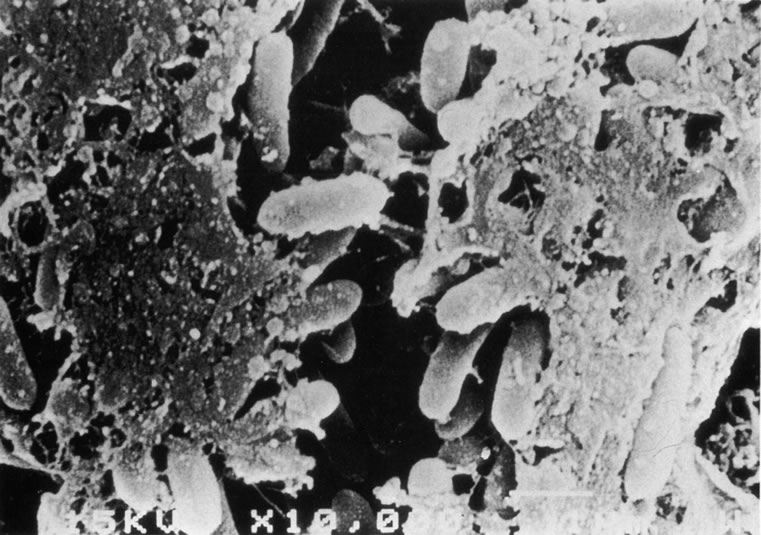
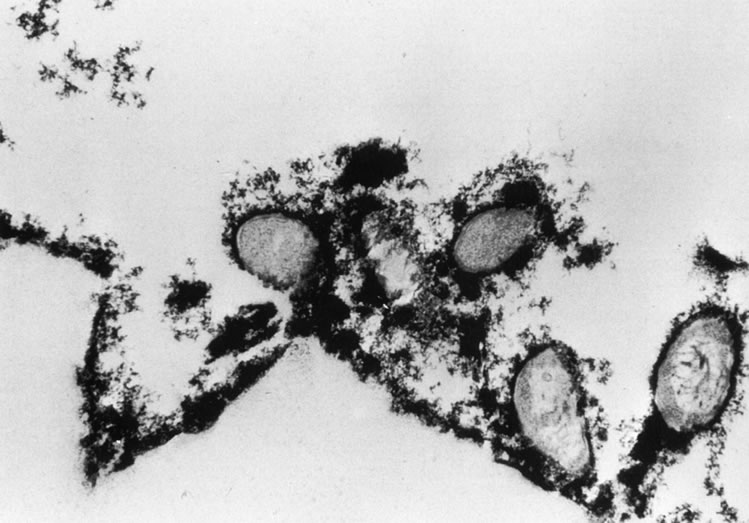
It has been shown in vitro that Pseudomonas will develop a bacterial glycocalyx, illustrated in Figure 7, within an hour of initial adherence to a lens surface.188,192 Bacterial adherence is of importance in retaining viable bacteria long enough on the lens surface for a glycocalyx to form. Subsequent to this, adhesion to the surface may be of little significance. The glycocalyx on a lens surface should not be regarded as isolated from the cornea; the relationship of lens glycocalyx to corneal/bacterial glycocalyx may be important, as P. aeruginosa has been shown to be capable of developing its own glycocalyx on other mucosal surfaces. However, if a corneal surface bacterial glycocalyx can develop, it may be of limited importance in the promotion of keratitis, as models of CL infection have not resulted in keratitis without an epithelial defect193 or severe hypoxia,130 although more subtle damage to the surface layers of epithelium has been shown to enhance adherence.194,195,202,203
The presence of bacterial biofilms on CLs provides one explanation for the pathogenesis of the keratitis that occurs in subjects whose CL cases and solutions are not contaminated by bacteria, and in compliant disposable EW SCL users. In these individuals, bacteria arising in small numbers from the environmental sources may adhere to the lens, whereas in the normal eye they are cleared by the ocular surface defense mechanisms. Pseudomonas has been demonstrated in over 6% of conjunctival cultures from asymptomatic individuals.134 If these adherent bacteria develop a glycocalyx and colonize the lens surface, their numbers can then amplify on the lens itself, increasing the likelihood of infection. This has been demonstrated in keratitis patients.196
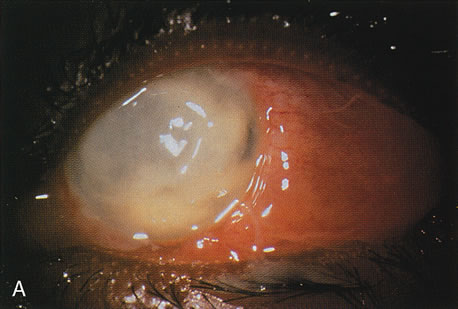
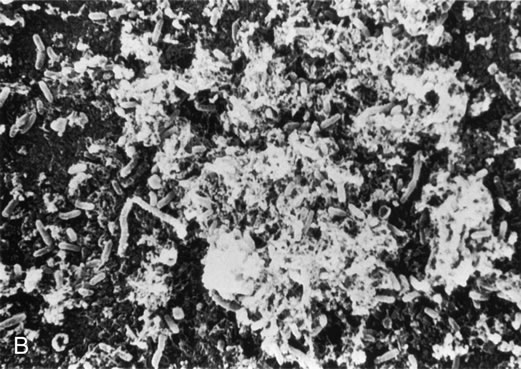
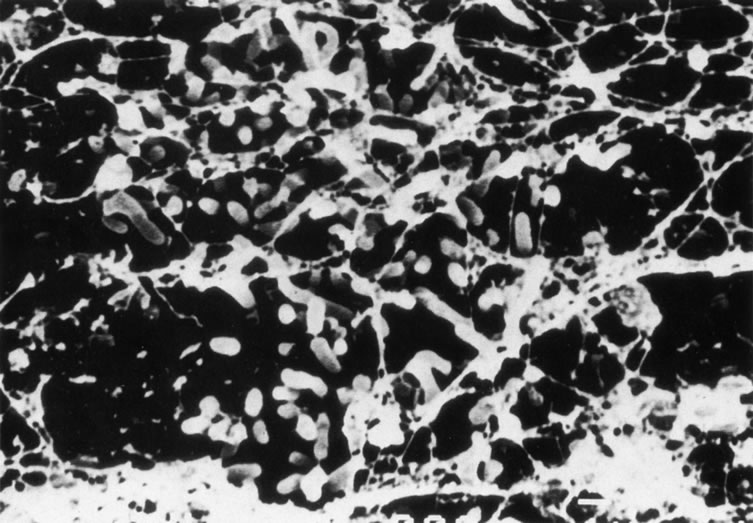
Figure 8A shows the cornea of a patient with Pseudomonas keratitis; in Figure 8B, the surface of her lens is shown to be contaminated by a bacterial biofilm. Figure 9 shows transmission electron microscopy of a bacterial biofilm from the lens of another patient with Pseudomonas keratitis. Bacterial biofilm is involved in the persistence of organisms in CL cases,137,190 as shown in Figure 10.
|
One study of biofilms of CLs and CL cases from patients with MK has shown biofilms in 17/20 CL cases and 11/20 CLs and no correlation with lens hygiene practice; this is to be expected as a result of the resistance of bacterial biofilms to conventional hygiene systems.191 Currently, CL disinfectant systems are tested against organisms in planktonic growth only; this is inadequate in view of the data now available on the ubiquity of bacterial biofilms in CL cases and on CLs.
The Role of the Contact Lens in Reducing Corneal Resistance to Bacterial Invasion of the Cornea
The profound effects on corneal oxygen supply resulting from all CL wear (and EW SCLs in particular), the disturbance of the normal flow of tears over the cornea, and the interference with the normal lid/tear resurfacing mechanisms are among the mechanical and physiologic stresses that may lead to a reduction in the clearance of bacteria contaminating the tear film. These factors may also lead to breaches in the corneal epithelium, common in CL users and necessary for the development of keratitis.74 Most studies of bacterial invasion of the cornea have used Pseudomonas and have shown that an epithelial break is necessary for the development of keratitis146,197–199 and that the bacteria adhere both to the injured epithelium,194 where the presence of glycosphingolipids may act as receptors for Pseudomonas,200 and to the exposed stroma.193,197 It is probable that several other factors are also involved in the adherence of this organism. It has been shown that fibronectin may inhibit Pseudomonas adherence201 and has been proposed that CL wear may promote adherence to the cornea by interfering with corneal cell surface fibronectin.202
A series of recent experiments has related the adhesion (binding) of P. aeruginosa to corneal epithelial cells in both the rabbit model and in humans. In the rabbit model adhesion of P. aeruginosa to the cornea was shown to parallel that to desquamated corneal epithelial cells, and the levels of adhesion could be related to the extended wear of CLs.195 Later experiments by the same group on P. aeruginosa binding to human desquamated corneal epithelial cells have demonstrated good correlation between binding and the use of daily and extended wear RGP SCLs, hydrogel SCLs and silicone/hydrogel SCLs.203,204 This model may be a good predictor of the potential for CLs to cause MK. It is one of the few models that has investigated RGP SCLs. Although RGP SCLs were associated with more epithelial morphologic changes than hydrogel or silicone/hydrogel CLs, their use did not alter the binding of P. aeruginosa to desquamated epithelial cells, correlating with the low incidence of MK found with this lens modality. Silicone/hydrogel lenses had a lesser effect on this measure of the potential for keratitis than hydrogel CLs. Interestingly, the binding of organisms returned to normal after 12 months which, if this is a real predictor of the potential for the development of keratitis, was anticipated to relate to a reduced risk with time. However, two of the epidemiologic studies4,108 did not demonstrate that period of wear was a factor associated with keratitis; these studies may not have had the power to show a small effect, and it remains to be seen how well this in vitro test correlates with keratitis risk.
The findings of these in vitro binding studies correlate with the cell biology of bacterial adherence to epithelial cells. Pili are important in Pseudomonas adherence and have been shown to bind to corneal epithelial cells in an in vitro model and in a buccal cell model in which binding was inhibited by pretreatment with purified pili or anti-pili antibody. After adherence of Pseudomonas, the bacteria rapidly disappear from the surface of the cornea. It is possible that the bacterial and epithelial cell membranes interact, resulting in invasion by the bacterium.145 Recent studies have shown that some strains of Pseudomonas are able to invade cells,204 whereas others are cytotoxic, causing cell necrosis and capable of damaging uninjured corneal epithelium in vitro.205 In addition, migrating epithelial cells bind more lectins than uninjured cells206; these migrating cells demonstrate similar characteristics to basal epithelial cells, to which Pseudomonas may adhere more readily when exposed after trauma. The low risk of keratitis in RGP CL users may be explained by differences in the presence of bacterial receptors on corneal epithelial cells.203
In experimental animal models of Pseudomonas, corneal infection keratitis will not occur in the presence of a contaminated CL unless there is epithelial trauma.189,194 However, suturing the lids together for a prolonged period will result in keratitis in the absence of a mechanical defect in the epithelium, probably as a result of the effects of severe hypoxic stress on epithelial resistance to infection.130,151,207 These findings correlate with the epidemiologic findings of increased risk associated with the overnight wear of hydrogel SCLs.
PATHOGENESIS OF ACANTHAMOEBA KERATITIS
The incidence of Acanthamoeba keratitis is less than that of bacterial keratitis, and its pathogenesis is less well understood, although the role of the contact lens in the pathogenesis of the infection is common to both. Like Pseudomonas, the organisms are widely distributed in the environment. Acanthamoeba is found in air, soil, and salt, fresh, and chlorinated water and can often be isolated from the nasopharyngeal cultures of human upper respiratory tract infections.208,209 In Japan, Acanthamoeba has been isolated from between one half and three quarters of soil samples in three cities and it has been suggested that direct contamination may be more important than CL case contamination. In the UK, Acanthamoeba has been isolated in association with lime-scale from domestic taps in hard water areas, particularly from tank-stored water, common in the UK.107 Subsequent studies confirmed that this was a risk factor for Acanthamoeba keratitis, with 6/27 patients with the disease having identical isolates of Acanthamoeba in their water supply and their eyes,48 correlating with the findings from an incidence study that demonstrated increased numbers of patients living in hard water areas in the UK.1 It seems likely that stored water is the principle source of Acanthamoeba isolated from home-made saline solutions47 and from the CL cases of CL users with Acanthamoeba keratitis.210 These organisms have also been isolated from the CL cases of 4% to 7% of asymptomatic CL users.132,139
Acanthamoeba is almost always present in the CL case environment of patients developing keratitis, as a co-contaminant with bacteria and/or fungi, which are required as a food source for the protozoan. Bacteria have been shown to have different capacities for enhancing both the growth of amoebae211 and their motility.210 Heat disinfection is effective against both Acanthamoeba cysts and trophozoites213,214 and has been advocated215 to provide protection against contamination with this organism. However, heat is not appropriate for use with all CL systems and, despite its in vitro effect, Acanthamoebae have been isolated from the CL cases of individuals using this method.139
Cold CL disinfection systems are not yet required to be effective against Acanthamoeba by the licensing authorities in the UK or U.S. Evaluation of the effect of these disinfection systems on Acanthamoeba trophozoites and cysts has had inconsistent results, possibly due to variations in the methodology, inoculum size, and species or strain susceptibility.216 Few of the systems available are effective when used for the minimum recommended disinfection times for bacteria. Hydrogen peroxide 3% may be effective against Acanthamoeba,217 but 2 to 4 hours217,218 of exposure is required. Peroxide systems that are inactivated within minutes of exposure are ineffective.211,214,218 One study of the susceptibility of Acanthamoeba culbertsonii has shown resistance to 24-hour soaking in hydrogen peroxide 3%,216 Chlorhexidine 0.004% and 0.005% is effective against the organism but also requires a prolonged exposure time219,221 and is susceptible to inactivation by organic debris, which reduces its efficacy.215 Thimerosal has been shown to have variable effects against Acanthamoeba species,213,216,218,220 but has been withdrawn because of the development of keratoconjunctivitis (see Table 2). Chlorine solutions are unlikely to have any effect against Acanthamoeba cysts at the concentrations available in CL solutions215,220; the organism survives in chlorinated tap water and swimming pools,222 and this disinfectant has now been withdrawn following epidemiologic studies demonstrating that it is associated with higher risks for both Acanthamoeba87 and bacterial keratitis.108 The newer CL disinfectants containing polyquaternary ammonium and polyhexamethylene biguanide are ineffective against Acanthamoeba in the concentrations used.203,218–220,222 As a response to the problems of Acanthamoeba keratitis, a new cold disinfection system,, containing myristamidopropyl dimethylamine (MAPD) has been developed to have some activity against Acanthamoeba.223 This system, together with hydrogen peroxide 3% and certain chlorhexidine preparations, are effective after overnight exposure.
Because of its requirement for bacteria as a food source, successful disinfection of bacterial contaminants would reduce or eliminate the problem of Acanthamoeba contamination but, as has already been discussed, this is currently not achieved in practice in 35% to 50% of asymptomatic CL users. These individuals are likely to be at a higher risk for Acanthamoeba keratitis if they are using a disinfection system that has no effect against the Acanthamoeba that may coexist in contaminated CLs cases. Like bacteria, Acanthamoeba cysts and trophozoites have also been shown to adhere to CLs,224 although they can be effectively removed by CL cleaners in vitro.225 Of these cleaners, isopropyl alcohol has been shown to be very effective against both cysts and trophozoites.216,220
The potential for the development of biofilms incorporating Acanthamoeba and bacteria has not been explored but it is probable, by analogy with the situation for bacteria, that biofilms may be important in the persistence of amoebae in CL cases and on lenses. Because of the difficulty of disinfecting lens systems of Acanthamoeba, preventing contamination is of greater importance for this organism than for bacteria. Measures that may protect against contamination include avoiding the use of tap water for cleaning lens cases or lenses, avoiding home-made solutions, cleaning the CL with a surfactant cleaner, drying the CL case after use, and the use of heat or solutions containing MAPD, chlorhexidine or peroxide with overnight soaking of CLs.
Specific adherence mechanisms have been shown for Acanthamoebae to rabbit corneal epithelium,226 and it is probable that these are also important in the pathogenesis of keratitis in humans. However, unlike bacteria, amoebae may be able to invade the corneal epithelium in the absence of an epithelial defect, as shown in vitro for human corneal epithelium227,228 and in a pig model of Acanthamoeba keratitis.229
The rarity of the infection, and the frequency with which CL users may be exposed to Acanthamoeba, implies that most strains of the organism are of low virulence in the cornea. Co-infection with bacteria has been shown to be necessary to establish Acanthamoeba keratitis in a rat model,230 and this may be paralleled in some cases of human keratitis in which co-infection with bacteria has been reported.49 Interstrain variability is almost certainly of importance, both in the ability of the organism to invade as well as its potential to establish keratitis once it has done so. Proteinase production has been shown to differ between pathogenic and nonpathogenic strains,231 and factors such as this are likely to partially determine virulence in the cornea.