In animals, mollicutes colonize mucous membranes and are therefore found in the ororespiratory and genitourinary tracts, often behaving as commensals. Mollicutes also infect the conjunctiva, the external ear, and the mammary glands of some animals. They tend to be host-specific. From their initial site of colonization, mollicutes may disseminate to various other body sites, particularly in immunodeficient patients.27,28 In humans who are immunocompetent, some mollicutes are isolated almost entirely from a particular site (e.g., M. pneumoniae from the ororespiratory tract), whereas others have a preference for one site but are also found in another (see Table 2).
Several mollicutes are human commensals without proven pathogenicity. The association of these mollicutes with disease complicates the diagnosis and necessitates extensive corroborative and highly specific serologic, nucleic acid, and epidemiologic data. They have been implicated as causes of acute and chronic disease at multiple sites and as cofactors in other diseases. Among these are M. salivarium and Mycoplasma orale, found most commonly in the oropharynx. M. penetrans has been isolated from urine in homosexual men and isolated with HIV infection and with Kaposi sarcoma; its pathogenicity awaits further study.29
Many mollicutes exhibit filamentous or flask-shaped appearances and display prominent and specialized polar tip organelles that mediate attachment to host target cells.1 These tip structures are complex, composed of a network of interactive proteins, designated adhesins, and adherence-accessory proteins. These proteins cooperate structurally and functionally to mobilize and concentrate adhesins at the tip and permit mollicute colonization of mucous membranes and eukaryotic cell surfaces, probably through host sialoglycoconjugates and sulfated glycolipids. Mollicute cytadherence-related proteins represent a superfamily of genes and proteins that have been conserved through horizontal gene transfer from an ancestral gene family.
Other mollicute species lack distinct tip structures but are capable of cytadherence, and they may use related genes and proteins or alternative mechanisms of surface parasitism.1 An interesting feature of specific M. pneumoniae and Mycoplasma genitalium adhesins is their multiple gene copy nature. Multiple truncated and sequence-related copies of the adhesin genes are dispersed throughout the genome, which could generate adhesin variation through homologous recombination. Despite their small genomes, pathogenic mollicutes facilitate DNA rearrangements through repetitive gene sequences, thus promoting genetic diversity and maximizing the coding potential of their limited genomes.1
Another characteristic of the cytadherencerelated proteins is their proline-rich composition, which markedly influences protein folding and binding. One of the most unusual features of the adhesins is their extensive sequence homology to mammalian structural proteins, with this molecular mimicry possibly provoking an anti-self response that triggers immune disorders. Patients with documented M. pneumoniae respiratory infections demonstrate seroconversion to myosin, keratin, and fibrinogen and exhibit extrapulmonary manifestations (Table 4).30 Mollicute adhesins exhibit amino acid sequence homologies with human CD4 and class II major histocompatibility complex lymphocyte proteins; this could generate attractive antibodies and trigger cell killing and immunosuppression.31 Mollicutes may serve as B-cell and T-cell mitogens and induce autoimmune disease through the activation of anti-self T cells or polyclonal B cells. The multiorgan, protean manifestations are consistent with the pathogenesis of autoimmunity and the induction of a broad range of immunoregulatory events, mediated by cytokine production. The direct effects on macrophages, B and T cells, and glial cells are evidence that mollicutes have the attributes of a primary mediator of disease.32
TABLE 59-4. Extrapulmonary Sequelae of Mycoplasma pneumoniae Infection
| System or Organ | Manifestations | Estimated Frequency |
| Cardiovascular | Myocarditis, pericarditis | <5% |
| Dermoid | Erythema multiforme, Stevens-Johnson syndrome, other rashes | Some skin involvement in ~25% |
| Gastrointestinal | Anorexia, nausea, vomiting, transient diarrhea | 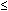 5% 5% |
| Hepatitis | ? | |
| Pancreatitis | ? | |
| Genitourinary | Tubo-ovarian abscess | Very low (insignificant) |
| Hematologic | Cold hemagglutinin production | ~50% |
| Hemolytic anemia | ? | |
| Thrombocytopenia | ? | |
| Intravascular coagulation | Very low (few cases reported) | |
| Musculoskeletal | Myalgia, arthralgia | 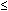 45% 45% |
| Arthritis | ? | |
| Nervous | Meningitis, meningoencephalitis, ascending paralysis, transient myelitis, cranial nerve palsy, and poliomyelitis-like illness | 6%–7% |
| Kidneys | Acute glomerulonephritis | ? |
Cytadherence is the initial step in the virulence process of pathogenic mollicutes and precedes a spectrum of subtle or overt host cell responses. In specific instances, distinct cytopathology correlates with the infecting mollicute species, the number of adherent mollicutes, the length of coincubation, the induction of proinflammatory cytokines, and the age and immune status of the patient; in other cases, however, there is no correlation of symptoms of disease and mollicute infection.12,16
Potent toxins have not been associated with mollicutes. The biologic properties of mollicutes that have been implicated as virulence determinants include:
- Generation of hydrogen peroxide and superoxide radicals by adhering mollicutes, causing
oxidative damage to the host cell membrane
- Competition for and depletion of nutrients or biosynthetic precursors by
mollicutes
- Existence of capsule-like material and electron-dense surface layers or
structures
- High-frequency phase and antigenic secretion, which results in surface
diversity and avoidance of protective host immune defenses
- Secretion or introduction of mollicute enzymes, such as phospholipases, ATPases, hemolysins, proteases, and nucleases into the host cell milieu
- Intracellular residence, which sequesters mollicutes and may establish
latent or chronic states.1,33
- The close contact with human membranes may also lead to fusion or exchange
of membrane components.
Whether pathogenic mollicutes enter and survive within mammalian cells has been debated for years. Two mycoplasmas are known to penetrate human cells (M. penetrans and M. fermentans). Sighting of intact mollicutes throughout the cytoplasm and the perinuclear regions of tissue cells from infected patients and in cell cultures and evidence that mollicutes are capable of long-term intracellular survival and replication in vitro offer an additional dimension to the pathogenic potential of mollicutes.34,35
Although there are multiple virulence strategies that may be displayed by mollicutes, there is no obvious single or group of mollicute properties that inextricably correlates with disease manifestations. Further research, specific microbiologic, epidemiologic, and diagnostic criteria, and detailed descriptions of biochemical, genetic, and immunologic characteristics that distinguish virulent and avirulent mollicutes are required. The development of experimental animal models is also important. Until then, there will continue to be a debate as to whether mollicutes are singular agents of infectious diseases, putative cofactors in the progression of other diseases, or universal contaminants of cell cultures.