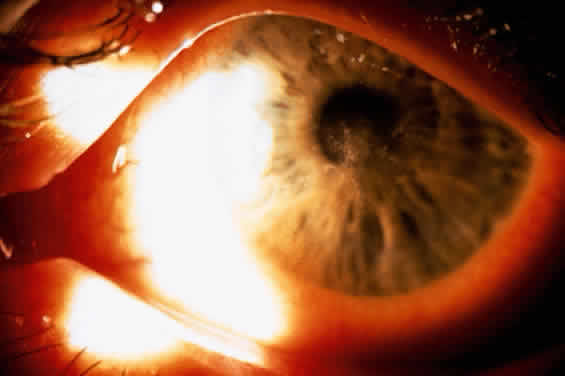
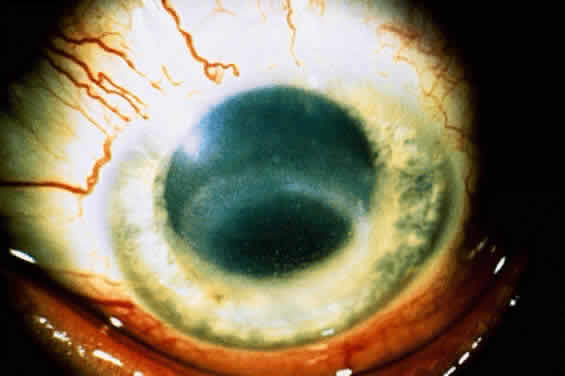
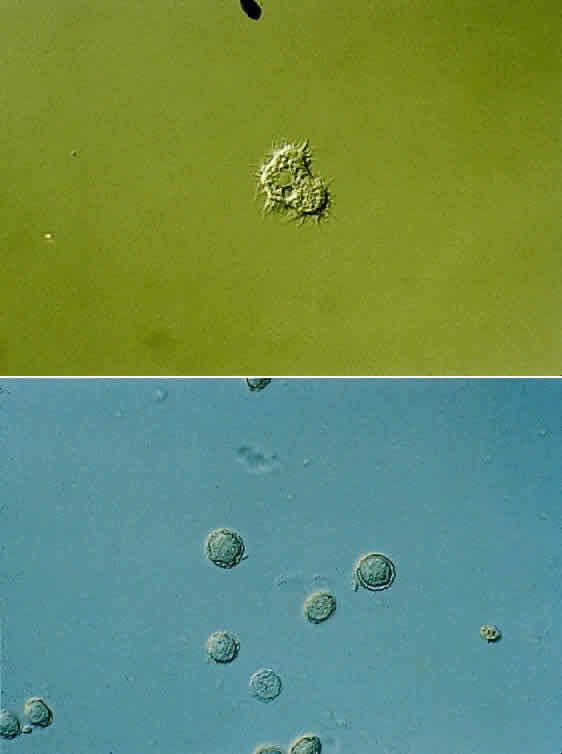
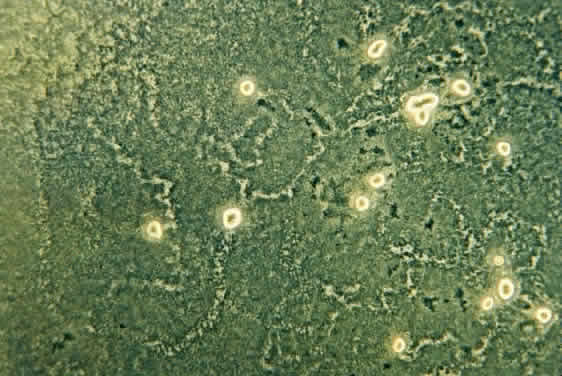
1. Culbertson CG: The pathogenicity of soil amoebas. Ann Rev Microbiol 25:231, 1971 2. Culbertson CG, Ensminger PN, Overton WM: Hartmannella (Acanthamoeba): Experimental chronic, granulomatous brain infections produced by new
isolates of low virulence. Am J Clin Pathol 46:305, 1966 3. Martinez AJ: Free-Living Amoebas: Natural History, Prevention, Diagnosis, Pathology, and
Treatment of Diseases. Boca Raton, FL, CRC Press, 1985 4. Martinez AJ: Infection of the central nervous system due to Acanthamoeba. Rev Infect Dis 13(suppl):399S, 1991 5. Martinez AJ: Free-living amebas: infection of the central nervous system. Mt Sinai J Med 60:271, 1993 6. Visvesvara GS, Stehr-Green JK: Epidemiology of freeliving ameba infections. J Protozool 37(suppl):25S, 1990 7. Visvesvara GS, Schuster FL, Martinez AJ: Balamuthia mandrillaris, N. G., N. Sp., agent of amebic meningoencephalitis in humans and other
animals. J Eukaryot Microbiol 40:504, 1993 8. Uberlaker JE: Acanthamoeba spp: “Opportunistic pathogens.”Trans Am Microsc Soc 110:289, 1991 9. Visvesvara GS: Classification of Acanthamoeba. Rev Infect Dis 13(suppl 5):S369, 1991 10. Visvesvara GS: Pathogenic and opportunistic free-living amebae. In Murray
PR, Baron EJ, Pfaller MA et al (eds): Manual of Clinical Microbiology, 6th
ed, p 1196. Washington, DC, American Society for Microbiology, 1995 11. Ma P, Visvesvara GS, Martinez AJ et al: Naegleria and Acanthamoeba infections: review. Rev Infect Dis 12:490, 1990 12. Martinez AJ: Acanthamoebiasis and immunosuppression: a case report. J Neuropathol Exp Neurol 41:548, 1982 13. Gonzalez MM, Gould E, Dickinson G et al: Acquired immunodeficiency syndrome associated with Acanthamoeba infection and other opportunistic organisms. Arch Pathol Lab Med 110:749, 1986 14. Sison JP, Kemper CA, Loveless M et al: Disseminated Acanthamoeba infection in patients with AIDS: case report and review. Clin Infect Dis 20:1207, 1995 15. Slater CA, Sickel JZ, Visvesvara GS et al: Brief report: successful treatment of disseminated Acanthamoeba infection in an immunocompromised patient. N Engl J Med 331:85, 1994 16. Tan B, Weldon-Linne CM, Rhone DP et al: Acanthamoeba infection presenting as skin lesions in patients with the acquired immunodeficiency
syndrome. Arch Pathol Lab Med 117:1043, 1993 17. Bottone EJ: Free-living amoebas of the genera Acanthamoeba and Naegleria: an overview and basic microbiologic correlates. Mt Sinai J Med 60:260, 1993 18. Kilvington S, White DG: Acanthamoeba: biology, ecology and human disease. Rev Med Microbiol 5:12, 1994 19. Stehr-Green JK, Bailey TM, Visvesvara GS: The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol 107:331, 1989 20. Tachikawa T, Ishibashi Y, Fujisawa S et al: A nation-wide survey on the occurrence of amoebic keratitis in Japan. Nippon Ganka Gakki Zasshi 99:68, 1995 21. Wilhelmus KR: Introduction: the increasing importance of Acanthamoeba. Rev Infect Dis 13(suppl):367S, 1991 22. Ficker L, Hunter P, Seal D, Wright P: Acanthamoeba keratitis occurring with disposable contact lens wear. Am J Ophthalmol 108:453, 1989 23. Jones DB, Visvesvera GS, Robinson NM: Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with a fatal meningoencephalitis. Trans Ophthalmol Soc UK 95:221, 1975 24. Moore MB, McCulley JP, Luckenbach MD et al: Acanthamoeba keratitis associated with soft contact lenses. Am J Ophthalmol 100:396, 1985 25. Moore MB, McCulley JP, Newton C et al: Acanthamoeba keratitis: a growing problem in soft and hard contact lens wearers. Ophthalmology 94:1654, 1987 26. Mortality and Morbidity Weekly Report: Acanthamoeba keratitis associated
with contact lenses—United States. MMWR 35:405, 1986 27. Nagington J, Watson PG, Playfair TJ et al: Amoebic infection of the eye. Lancet 2:1537, 1974 28. Palmer ML, Hyundiuk RA: Contact lens-related infectious keratitis. Int Ophthalmol Clin 33:23, 1993 29. Auran JD, Starr MB, Jakobiec FA: Acanthamoeba keratitis: a review of the literature. Cornea 6:2, 1987 30. Singh BN: Nuclear division as the basis for possible phylogenic classification of
the order Amoebida Kent, 1880. Indian J Parasitol 5:133, 1981 31. Byers TJ: Growth, reproduction, and differentiation in Acanthamoeba. Int Rev Cytol 61:283, 1979 32. Lee JJ, Hutner SH, Bovee EC (eds): An Illustrated Guide to the Protozoa, p 211. Lawrence, KS, Allen Press, Society of Protozoologists, 1985 33. Castellani A: An amoeba found in cultures of yeast: preliminary note. J Trop Med Hyg 33:160, 1930 34. Pussard M: Le genre Acanthamoeba Volkonsky 1931 (Hartmanellidae-Amoebida). Protistologica 2:71, 1966 35. Singh BN, Hanumaiah V: Studies on the pathogenic and non-pathogenic amoebae
and the bearing of nuclear division and locomotive form and behaviour
on the classification of the order Amoebida. Monograph No. 1 of the
Association of Microbiologists of India, pp 1–80, 1979 36. Volkonsky M: Hartmannella castellanii Douglas et classification des hartmannelles. Arch Zool Exp Gen 72:317, 1931 37. Page FC: Re-definition of the genus Acanthamoeba with descriptions of three species. J Protozol 14:709, 1967 38. Page FC: A New Key to Freshwater and Soil Gymnamoebae, pp 1–122. Cumbria, England, Freshwater Biological Association, 1988 39. Visvesvara GS, Balamuth W: Comparative studies on related free-living and pathogenic amoebae with
special reference to Acanthamoeba. J Protozool 22:245, 1975 40. Pussard M, Pons R: Morphologie de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebida). Protistologica 3:557, 1977 41. Byers TJ, Hugo ER, Stewart VJ: Genes of Acanthamoeba: DNA, RNA and protein sequences (a review). J Protozool 37(suppl):17S, 1990 42. Gautom RK, Stephen L, Seyedirashti S et al: Mitochondrial DNA fingerprinting of Acanthamoeba spp. isolated from clinical and environmental sources. J Clin Microbiol 32:1070, 1994 43. Kilvington S, Beeching JR, White DG: Differentiation of Acanthamoeba strains from infected corneas and the environment by using restriction
endonuclease digestion of whole cell DNA. J Clin Microbiol 29:310, 1991 44. McLaughlin GL, Brandt FH, Visvesvara GS: Restriction fragment length polymorphisms of the DNA of selected Naegleria and Acanthamoeba amoebae. J Clin Microbiol 26:1655, 1988 45. Yagita K, Endo T: Restriction enzyme analysis of mitochondrial DNA of Acanthamoeba strains in Japan. J Protozool 37:570, 1990 46. De Jonckheere JF: Studies on pathogenic free-living amoebae in swimming pools. Bull Inst Pasteur 77:385, 1981 47. De Jonckheere JF: Ecology of Acanthamoeba. Rev Infect Dis 13(suppl):S385, 1991 48. Sawyer TK: Free-living pathogenic and nonpathogenic amoeba in Maryland soils. Appl Environ Microbiol 55:1074, 1989 49. Sawyer TK, Lewis EJ, Galasso M et al: Pathogenic amoebae in ocean sediments near waste water sludge disposal
sites. J Water Poll Contr Fed 54:1318, 1982 50. Kyle DE, Noblet GP: Vertical distribution of potentially pathogenic free-living amoebae in
freshwater lakes. J Protozool 32:99, 1985 51. Jones DB: Acanthamoeba: The ultimate opportunist? Am J Ophthalmol 102:527, 1986 52. Chynn EW, Lopez MA, Pavan-Langston D, Talamo JH: Acanthamoeba keratitis: contact lens and noncontact lens characteristics. Ophthalmology 102:1369, 1995 53. Lindquist TD, Doughman DJ, Rubenstein JB et al: Acanthamoeba-contaminated hydrogel contact lenses. Cornea 7:300, 1988 54. Ludwig IH, Meisler DM, Rutherford I et al: Susceptibility of Acanthamoeba to soft contact lens disinfection systems. Invest Ophthalmol Vis Sci 27:626, 1986 55. Florakis FJ, Solberg R, Krachmer JG et al: Elevated corneal epithelial lines in Acanthamoeba keratitis. Arch Ophthalmol 106:1202, 1988 56. Lindquist TD, Sher NA, Doughman DJ: Clinical signs and medical therapy of early Acanthamoeba keratitis. Arch Ophthalmol 106:73, 1988 57. Moore MB, McCulley JP, Kaufman HE, Robin JB: Radial keratoneuritis as a presenting sign of Acanthamoeba keratitis. Ophthalmology 93:1310, 1986 58. Holland GN, Donzis PB: Rapid resolution of early Acanthamoeba keratitis after epithelial débridement. Am J Ophthalmol 104:87, 1987 59. Mannis MJ, Tamaru R, Roth AM et al: Acanthamoeba sclerokeratitis: determining diagnostic criteria. Arch Ophthalmol 104:1313, 1986 60. McClellan K, Coster DJ: Acanthamoeba keratitis diagnosed by paracentesis and biopsy and treated with propamidine. Br J Ophthalmol 71:734, 1987 61. Theodore FH, Jakobiec FA, Juechter KB et al: The diagnostic value of a ring infiltrate in acanthamoebic keratitis. Ophthalmology 92:1471, 1985 62. Moore MB, McCulley JP: Acanthamoeba keratitis associated with contact lenses: six consecutive cases of successful
management. Br J Ophthalmol 73:271, 1989 63. Scully RE, Mark ES, McNeely BU: Case records of the Massachusetts General Hospital: weekly clinicopathological
exercises: case 10-1985. N Engl J Med 312:634, 1985 64. Wessely K: Ueber anaphylaktische Erscheinungen an der Hornhaut. München Med Wochenschr 58:1713, 1911 65. Mondino BJ, Rabin BS, Kessler E et al: Corneal rings with gram-negative bacteria. Arch Ophthalmol 95:2222, 1977 66. Lindquist TD, Fritsche TR, Grutzmacher RD: Scleral ectasia secondary to Acanthamoeba keratitis. Cornea 9:74, 1990 67. Ashton N, Stamm W: Amoebic infection of the eye: a pathological report. Trans Ophthalmol Soc UK 95:214, 1975 68. Key SN III, Green WR, Willaert E et al: Keratitis due to Acanthamoeba castellanii: a clinicopathologic case report. Arch Ophthalmol 98:475, 1980 69. Knox DL, Bayless TM: Gastrointestinal and ocular disease. In Mausolf FA (ed): The
Eye in Systemic Disease, pp 274–285. St. Louis, CV Mosby, 1975 70. Bos HJ, Volker-Dieben JHM, Kok-van Alphen CC: A case of Acanthamoeba keratitis in the Netherlands. Trans R Soc Trop Med Hyg 75:86, 1981 71. Hamburg A, De Jonckheere JF: Amoebic keratitis. Ophthalmologica 181:74, 1980 72. Lund OE, Stefani FH, Dechant W: Amoebic keratitis: a clinicopathologic case report. Br J Ophthalmol 62:373, 1978 73. Ma P, Willaert E, Juechter KB, Stevens AR: A case of keratitis due to Acanthamoeba in New York, New York, and features of 10 cases. J Infect Dis 143:662, 1981 74. Nagington J: Isolation of amoebae from eye infections in England. Trans Ophthalmol Soc UK 95:207, 1975 75. Samples JR, Binder PS, Luibel FJ et al: Acanthamoeba keratitis possibly acquired from a hot tub. Arch Ophthalmol 102:707, 1984 76. Sawyer TK, Visvesvara GS, Harke BA: Pathogenic amoebas from brackish and ocean sediments, with a description
of Acanthamoeba hatchetti, n. sp. Science 196:1324, 1977 77. Warhurst DC, Thomas SC: Acanthamoeba spp. from corneal ulcers (abstr). J Protozool 22:57A, 1975 78. Warhurst DC, Stamm WP, Phillips EA: Acanthamoeba from a new case of corneal ulcer. Trans R Soc Trop Med Hyg 70:279, 1976 79. Watson PG: Amoebic infection of the eye. Trans Ophthalmol Soc UK 95:204, 1975 80. Blackman HJ, Rao NA, Lemp MA, Visvesvara GS: Acanthamoeba keratitis successfully treated with penetrating keratoplasty: suggested
immunogenic mechanism of action. Cornea 3:125, 1984 81. Cohen EJ, Buchanan HW, Laughrea PA et al: Diagnosis and management of Acanthamoeba keratitis. Am J Ophthalmol 100:389, 1985 82. Cohen EJ, Parlato CJ, Arentsen JJ et al: Medical and surgical treatment of Acanthamoeba keratitis. Am J Ophthalmol 103:615, 1987 83. Dornic DI, Wolf T, Dillon WH et al: Acanthamoeba keratitis in soft contact lens wearers. J Am Optom Assoc 58:482, 1987 84. Hirst LW, Green WR, Merz W et al: Management of Acanthamoeba keratitis: a case report and review of the literature. Ophthalmology 91:1105, 1984 85. Stehr-Green JK, Bailey TM, Brandt FH et al: Acanthamoeba keratitis in soft contact lens wearers: a case-control study. JAMA 258:57, 1987 86. Donzis PB, Mondino BJ, Weissman BA, Bruckner DA: Microbial contamination of contact lens care systems. Am J Ophthalmol 104:325, 1987 87. Larkin DFP, Kilvington S, Easty DL: Contamination of contact lens storage cases by Acanthamoeba and bacteria. Br J Ophthalmol 74:133, 1990 88. Gray TB, Cursons RTM, Sherwan JF, Rose PR: Acanthamoeba, bacterial, and fungal contamination of contact lens storage cases. Br J Ophthalmol 79:601, 1995 89. Devonshire P, Munro FA, Abernethy C, Clark BJ: Microbial contamination of contact lens cases in the west of Scotland. Br J Ophthalmol 77:41, 1993 90. Seal D, Stapleton F, Dart J: Possible environmental sources of Acanthamoeba spp in contact lens wearers. Br J Ophthalmol 76:424, 1992 91. Bottone EJ, Madayag RM, Qureshi MN: Acanthamoeba keratitis: synergy between amoebic and bacterial cocontaminants in contact
lens care systems as a prelude to infection. J Clin Microbiol 30:2447, 1992 92. Qureshi MN, Perez AA II, Madayag RM, Bottone EJ: Inhibition of Acanthamoeba species by Pseudomonas aeruginosa: rationale for their selective exclusion in corneal ulcers and contact
lens care systems. J Clin Microbiol 31:1908, 1993 93. John T, Desai D, Sahm D: Adherence of Acanthamoeba castellanii cysts and trophozoites to unworn soft contact lenses. Am J Ophthalmol 108:658, 1989 94. Kilvington S: Acanthamoeba trophozoite and cyst adherence to four types of soft contact lens and
removal by cleaning agents. Eye 7:535, 1993 95. Perkovich BT, Meisler DM, McMahon JT, Rutherford I: Acanthamoeba adherence to soft contact lenses. Invest Ophthalmol Vis Sci 30(suppl):198, 1989 96. Badenoch PR, Grimmond TR, Cadwgan J et al: Nasal carriage of free-living amoebae. Microbiol Ecol Health Dis 1:209, 1988 97. De Jonckheere JF, Michel R: Species identification and virulence of Acanthamoeba strains from human nasal mucosa. Parasitol Res 74:314, 1988 98. Lawande RV, Abraham SN, John I, Egler LJ: Recovery of soil amebas from the nasal passages of children during the
dusty harmattan period in Zaria. Am J Clin Pathol 71:201, 1979 99. Wang SS, Feldman HA: Isolation of Hartmannella species from human throats. N Engl J Med 277:1174, 1967 100. Taylor WM, Pidherney MS, Alizadeh H, Niederkorn JY: In vitro characterization of Acanthamoeba castellanii cytopathic effect. J Parasitol 31:603, 1995 101. Badenoch PR: The pathogenesis of Acanthamoeba keratitis. Austral NZ J Ophthalmol 19:9, 1991 102. Morton LD, McLaughlin GL, Whiteley HE: Effects of temperature, amebic strain, and carbohydrates on Acanthamoeba adherence to corneal epithelium in vitro. Infect Immun 59:3819, 1991 103. Uberlaker JE, Moore MB, Martin JH et al: In vitro intercellular adherence of Acanthamoeba castellanii: a scanning and transmission electron microscopy study. Cornea 10:299, 1991 104. Diaz J, Osuna A, Rosales MJ et al: Sucker-like structures in two strains of Acanthamoeba: scanning electron microscopy study. Intl J Parasitol 21:365, 1991 105. Larkin DFP, Berry M, Easty DL: In vitro corneal pathogenicity of Acanthamoeba. Eye 5:560, 1991 106. Badenoch PR, Adams M, Coster DJ: Corneal virulence, cytopathic effect on human keratocytes and genetic characterization
of Acanthamoeba. Int J Parasitol 25:229, 1995 107. Garner A: Pathogenesis of acanthamoebic keratitis: hypothesis based on analysis of 30 cases. Br J Ophthalmol 77:366, 1993 107a. Moore MB, Ubelaker JE, Martin JH et al: In vitro penetration of human corneal epithelium by Acanthamoeba castellanii: a scanning and transmission electron microscopy study. Cornea 10:291, 1991 108. Pellegrin J-LJ, Ortega-Barria E, Barza M et al: Neuraminidase activity in Acanthamoeba species trophozoites and cysts. Invest Ophthalmol Vis Sci 32:3061, 1991 109. van Klink F, Alizadeh H, Stewart GL et al: Characterization and pathogenic potential of a soil isolate and an ocular
isolate of Acanthamoeba castellani in relation to Acanthamoeba keratitis. Curr Eye Res 11:1207, 1992 110. Larkin DFP, Easty DL: External eye flora as a nutrient source for Acanthamoeba. Graefes Arch Clin Exp Ophthalmol 228:458, 1990 111. Badenoch PR, Christy PE, Johnson AM, Coster DJ: Pathogenicity of Acanthamoeba and a corynebacterium in the rat cornea. Arch Ophthalmol 108:107, 1990 112. Badenoch PR, Johnson AM, Christy PE, Coster DJ: A model of Acanthamoeba keratitis in the rat. Rev Infect Dis 13(suppl):S445, 1991 113. Fritsche TR, Gautom RK, Seyedirashti S et al: Occurrence of bacterial endosymbionts in Acanthamoeba spp. isolated from corneal and environmental specimens and contact lenses. J Clin Microbiol 31:1122, 1993 114. Gautom RK, Fritsche TR: Transmissibility of obligate bacterial endosymbionts between isolates of Acanthamoeba spp. J Euk Microbiol 42:452, 1995 115. Hall J, Voelz H: Bacterial endosymbionts of Acanthamoeba sp. J Parasitol 71:89, 1985 116. Michel R, Hauroder-Philippczyk B: Acanthamoeba from human nasal mucosa infected with an obligate intracellular parasite. Eur J Protistol 30:104, 1994 117. Yagita K, Matias RR, Yasuda T et al:Acanthamoeba sp. from the Philippines: electron microscopy study on naturally occurring
bacterial symbionts. Parasitol Res 81:98, 1995 118. Wilhelmus KR, Osato MS, Font RL et al: Rapid diagnosis of Acanthamoeba keratitis using calcofluor white. Arch Ophthalmol 104:1309, 1986 119. Rivasi F, Longanesi L, Casolar C et al: Cytologic diagnosis of Acanthamoeba keratitis: report of a case with correlative study with indirect immunofluorescence
and scanning electron microscopy. Acta Cytol 39:821, 1995 120. Singh BN: A culture method for growing small free-living amoebae and for the study
of their nuclear division. Nature Lond 165:65, 1950 121. Auran JD, Starr MB, Koester CJ, LaBombardi VJ: In vivo scanning slit confocal microscopy of Acanthamoeba keratitis: a case report. Cornea 13:183, 1994 122. Irvine JA, Ariyasu R: Limitation in tandem scanning confocal microscopy as a diagnostic tool
for microbial keratitis. Scanning 16:307, 1994 123. Winchester K, Mathers WD, Sutphin JE, Daley TE: Diagnosis of Acanthamoeba keratitis in vivo with confocal microscopy. Cornea 14:1, 1995 124. Ishibashi Y, Matsumoto Y, Kabata T et al: Oral itraconazole and topical miconazole with débridement for Acanthamoeba keratitis. Am J Ophthalmol 109:121, 1990 125. Bacon AS, Dart JKG, Ficker LA et al: Acanthamoeba keratitis: the value of early diagnosis. Ophthalmology 100:1238, 1993 126. Bacon AS, Frazer DG, Dart JKG et al: A review of 72 consecutive cases of Acanthamoeba keratitis: 1984-1992. Eye 7:719, 1993 127. Illingworth CD, Cook SD, Karabatsas CH, Easty DL: Acanthamoeba keratitis: risk factors and outcome. Br J Ophthalmol 79:1078, 1995 128. Brooks JG Jr, Coster DJ, Badenoch PR: Acanthamoeba keratitis: resolution after epithelial débridement. Cornea 13:186, 1994 129. Elder MJ, Kilvington S, Dart JKG: A clinicopathologic study of in vitro sensitivity testing and Acanthamoeba keratitis. Invest Ophthalmol Vis Sci 35:1059, 1994 130. Hay J, Kirkness CM, Seal DV, Wright P: Drug resistance and Acanthamoeba keratitis: the quest for alternative antiprotozoal chemotherapy. Eye 8:555, 1994 131. Kuyyakanond T, Quesnel LB: The mechanism of action of chlorhexidine. FEMS Microbiol Lett 100:211, 1992 132. Seal DV, Hay J, Kirkness CM: Chlorhexidine or polyhexamethylene biguanide for Acanthamoeba keratitis. Lancet 345:136, 1995 133. Booth A, Morrell AJ: Letter to the Editor. Eye 8:719, 1994 134. Kilvington S: Letter to the Editor. Lancet 315:792, 1995 135. Larkin DFP, Kilvington S, Dart JKG: Treatment of Acanthamoeba keratitis with polyhexamethylene biguanide. Ophthalmology 99:185, 1992 136. Mills RA, Wilhelmus KR, Osato MS, Pyron M: Polyhexamethylene biguanide in the treatment of Acanthamoeba keratitis. Aust NZ J Ophthalmol 21:277, 1993 137. Varga JH, Wolf TC, Jensen HG et al: Combined treatment of Acanthamoeba keratitis with propamidine, neomycin, and polyhexamethylene biguanide. Am J Ophthalmol 115:466, 1993 138. Berry M, Easty DL: Isolated human and rabbit eye: models of corneal toxicity. Toxicol In Vitro 7:461, 1993 139. Archer HG, Barnett S, Irving S et al: A controlled model of moist wound healing: comparison between semi-permeable
film, antiseptics and sugar paste. J Exp Pathol 71:155, 1990 140. Green K, Livingston V, Bowman K, Hull DS: Chlorhexidine effects on corneal epithelium and endothelium. Arch Ophthalmol 98:1273, 1980 141. Elder MJ, Dart JKG: Chemotherapy for Acanthamoeba keratitis. Lancet 345:791, 1995 142. Seal DV, Hay J, Connor R, Cairns D: Guanidines, diamidines, and biguanides: Towards
a rational therapy for Acanthamoeba keratitis, p37 (abstr). Ocular Microbiology and Immunology Group, 29th
Annual Meeting, Atlanta, GA, October 28, 1995 143. Wright P, Warhurst D, Jones BR: Acanthamoeba keratitis successfully treated medically. Br J Ophthalmol 69:778, 1985 144. Berger ST, Mondino BJ, Hoft RH et al: Successful medical management of Acanthamoeba keratitis. Am J Ophthalmol 110:395, 1990 145. D'Aversa G, Stern GA, Driebe WT Jr: Diagnosis and successful medical treatment of Acanthamoeba keratitis. Arch Ophthalmol 113:1120, 1995 146. Kilvington S, Larkin DFP, White DG, Beeching JR: Laboratory investigation of Acanthamoeba keratitis. J Clin Microbiol 28:2722, 1990 147. Brasseur G, Favennec L, Perrine D et al: Successful treatment of Acanthamoeba keratitis by hexamidine. Cornea 13:459, 1994 148. Casemore DP: Sensitivity of Hartmannella (Acanthamoeba) to 5-fluorocystosine, hydroxystilbamidine and other substances. J Clin Pathol 23:649, 1970 149. Nagington J, Richards JE: Chemotherapeutic compounds and Acanthamoeba from eye infections. J Clin Pathol 29:648, 1976 150. Perrine D, Barbier D, Chenu P, Georges P: Comparative study of cysticidal
effects of three diamidines on Acanthamoeba strains isolated from keratitis (abstr 288). VIth International Conference
on the Biology and Pathogenicity of Free-Living Amoebae, 1992 151. Hugo ER, Byers TJ: S-adenosyl-l-methionine decarboxylase of Acanthamoeba castellanii (Neff): purification and properties. Biochem J 295:203, 1993 152. Greenidge PA, Jenkins TC, Neidle S: DNA minor groove recognition properties of pentamidine and its analogs: a
molecular modelling study. Mol Pharmacol 43:982, 1993 153. Jenkins TC, Lane AN, Neidle S, Brown DG: NMR and molecular modeling studies of the interaction of berenil and pentamidine
with d(CGCAAATTTGCG)2. Eur J Biochem 213:1175, 1993 154. Arnott MA, Hay J: The effect of pentamidine salts on the NADPH-oxidase system of stimulated
neutrophilic granulocytes. J Antimicrob Chemother 25:247, 1990 155. Arnott MA, Bennett ND, Cairns D, Hay J: Selective effects of pentamidine on cytosolic and granule-associated enzyme
release from zymosan-activated human neutrophilic granulocytes. J Pharm Pharmacol 46:394, 1994 156. Johns KJ, Head WS, O'Day DM: Corneal toxicity of propamidine. Arch Ophthalmol 106:68, 1988 157. Osato MS, Robinson NM, Wilhelmus KR, Jones DB: In vitro evaluation of antimicrobial compounds for cysticidal activity against Acanthamoeba. Rev Infect Dis 13(suppl 5):S431, 1991 158. Wilson FM II: Toxic and allergic reactions to topical ophthalmic medications. In
Arffa RC (ed): Grayson's Diseases of the Cornea, 3rd ed, p 632. St. Louis, Mosby-Year Book, 1991 159. Holland EJ, Alul IH, Meisler DM et al: Subepithelial infiltrates in Acanthamoeba keratitis. Am J Ophthalmol 112: 414, 1991 160. Driebe WT, Stern GA, Epstein RJ et al: Acanthamoeba keratitis. Arch Ophthalmol 106:1196, 1988 161. Epstein RJ, Wilson LA, Visvesvara GS, Plourde EG Jr: Rapid diagnosis of Acanthamoeba keratitis from corneal scrapings using indirect fluorescent antibody staining. Arch Ophthalmol 104:1318, 1986 162. Ficker L, Seal D, Warhurst D, Wright P: Acanthamoeba keratitis-resistance to medical therapy. Eye 4:835, 1990 163. Schuster FL: Comparative effects of selected azole compounds on trophic and cystic stages
of Acanthamoeba polyphaga. J Eukaryot Microbiol 40:563, 1993 164. Gilbert ML, Osato MS, Wilhelmus KR: Ocular toxicity of topical clotrimazole preparations. J Toxicol Cutan Ocular Toxicol 6:3, 1987 165. Saunders PPR, Proctor EM, Rollins DF, Richards JSF: Enhanced killing of Acanthamoeba cysts in vitro using dimethylsulfoxide. Ophthalmology 99:1197, 1992 166. Osato M, Robinson N, Wilhelmus K, Jones D: Morphogenesis of Acanthamoeba castellanii: titration of the steroid effect. Invest Ophthalmol Vis Sci 27(suppl):37, 1986 167. John T, Lin J, Sahm D, Rockey JH: Effects of corticosteroids in experimental Acanthamoeba keratitis. Rev Infect Dis 13(suppl):S440, 1991 168. Meisler DM, Ludwig IH, Rutherford I et al: Susceptibility of Acanthamoeba to cryotherapeutic methods. Arch Ophthalmol 104:130, 1986 169. Binder PS: Cryotherapy for medically unresponsive Acanthamoeba keratitis. Cornea 8:106, 1989 170. Stopak SS, Roat MI, Nauheim RC et al: Growth of Acanthamoeba on human corneal epithelial cells and keratocytes in vitro. Invest Ophthalmol Vis Sci 32:354, 1991 171. Matoba AY, Pare PD, Le TD, Osato MS: The effects of freezing and antibiotics on the viability of Acanthamoeba cysts. Arch Ophthalmol 107:439, 1989 172. Margo CE, Brinser JH, Groden L: Exfoliated cytopathology of Acanthamoeba keratitis. JAMA 255:2216, 1986 173. Ficker LA, Kirkness C, Wright P: Prognosis for keratoplasty in Acanthamoeba keratitis. Ophthalmology 100:105, 1992 174. CLAO: Contact lens care: new guidelines (CLAO policy statement issued April 1987). CLAO
J 14:55, 1988 175. Silvany RE, Doughtery JM, McCulley JP et al: The effect of currently available contact lens disinfection systems on Acanthamoeba castellanii and Acanthamoeba polyphaga. Ophthalmology 97:286, 1990 176. Silvany RE, Dougherty JM, McCulley JP: Effect of contact lens preservatives on Acanthamoeba. Ophthalmology 98:854, 1991 177. Kilvington S: Moist heat disinfection of Acanthamoeba cysts. Rev Infect Dis 13:418, 1991 |