TABLE ONE. Infectious Agents Associated with Ophthalmic Disease in HIV-Infected
Patients
- Infections of the retina and choroid
- Viruses
- Cytomegalovirus
- Herpes simplex virus
- Varicella zoster virus
- Cytomegalovirus
- Parasites
- Toxoplasma gondii
- Pneumocystis carinii
- Toxoplasma gondii
- Fungi
- Bipolaris hawaiiensis
- Candida albicans
- Cryptococcus neoformans
- Fusarium sp
- Histoplasma capsulatum
- Sporotrichum schenckii
- Bipolaris hawaiiensis
- Spirochetes
- Bacteria
- Endogenous bacteria
- Nocardia sp
- Endogenous bacteria
- Mycobacteria
- Mycobacterium avium complex
- Mycobacterium tuberculosis
- Mycobacterium avium complex
- Viruses
- Infections of the ocular surface and adnexa
- Viruses
- Herpes simplex virus
- Molluscum contagiosum
- Varicella zoster virus
- Herpes simplex virus
- Parasites
- Microsporidia
- Microsporidia
- Fungi
- Candida albicans
- Candida albicans
- Chlamydia
- Chlamydia trachomatis, serotype L2 (lymphogranuloma venereum)
- Chlamydia trachomatis, serotype L2 (lymphogranuloma venereum)
- Bacteria
- Staphylococcus sp
- Pseudomonas sp
- Capnocytophaga sp
- Staphylococcus sp
- Viruses
* Classified as a protozoan parasite, but recent evidence suggests it is more closely related to fungi.
TABLE TWO. Autopsy Findings in 35 AIDS Patients
| Pathogen | Ocular Infection (No. cases and %) | Nonocular Infection* (No. cases and %) |
| Cytomegalovirus | 12 (34) | 25 (71) |
| M. | 2 (6) | 12 (34) |
| avium-intracellulare | ||
| Cryptococcus neoformans | 2 (6) | 5 (14) |
| Herpes simplex virus | 1 (3) | 11 (31) |
| Pneumocystis carinii | 0 (33) | 26 (74) |
| Candida albicans | 0 (33) | 14 (40) |
| Herpes zoster virus | 0 (33) | 4 (11) |
| Toxoplasma gondii | 0 (33) | 3 (9) |
| Aspergillus sp | 0 (33) | 2 (6) |
| Nocardia sp | 0 (33) | 1 (3) |
Cytomegalovirus (CMV) retinopathy is by far the most common of the severe ophthalmic infections in patients with AIDS. Varicella zoster virus retinopathy is being seen with increasing frequency; although it is probably the second most common retinal infection in North American HIV-infected patients, it is still far less common than CMV retinopathy. Toxoplasmic retinochoroiditis is the third retinal infection that is seen with some regularity, especially in certain areas of Europe and South America. The remaining retinal infections associated with AIDS are uncommon. They are seen with disseminated infections, in which the massive proliferation of organisms may overwhelm the patient's limited immune defenses. They are usually seen late in the course of the syndrome; thus, they may actually signal further waning of immune defenses. Patients with intraocular infections should be suspected of having tissue-invasive infections involving other internal organs. When they do occur, intraocular infections are frequently extensive and severe.
Infections of the cornea and external eye are less common in HIV-infected individuals than intraocular infections. The natural defenses of normal eyelid function, tear film, and an intact corneal epithelium protect against many exogenous pathogens. It is therefore important that factors that might compromise those defenses, such as epithelial defects or trichiasis, be corrected as soon as possible. Also, HIV-infected patients who wear contact lenses should adhere strictly to lens care and disinfection techniques. Many HIV-infected individuals appear to have decreased aqueous tear production, which leads to feelings of dryness and irritation but does not seem to result in an increased incidence of ocular surface infections. At greater risk are patients who have permanent damage to the eyelids from zoster ophthalmicus, neurotrophic corneas from herpetic infections, or damage to the ocular surface from cicatricial conjunctivitis, similar to that of Stevens-Johnson syndrome, which is seen occasionally in patients with AIDS.
HIV infection and its sequelae are being studied in a variety of animal models. Rhesus monkeys infected with simian immunodeficiency virus develop an AIDS-like illness. In this model the monkeys develop a variety of ophthalmic lesions, including CMV infections of the retina.10 This model may be useful for future studies of AIDS-related ophthalmic infections.
CYTOMEGALOVIRUS
Cytomegalovirus retinopathy is the most thoroughly studied of the ophthalmic manifestations of HIV infection. CMV retinopathy is an AIDS-defining index disease. Most studies have concentrated on treatment, but a great deal has been learned about the natural history of CMV retinopathy during the course of these studies.
Cytomegalovirus is a ubiquitous DNA virus of the herpes group. Most of the adult population has been infected with CMV, but in most cases it does not cause any clinically apparent disease. The virus remains in the body, however, as a latent infection.
In patients with AIDS, CMV can cause life-threatening infections involving many tissues, including the brain, lungs, and gastrointestinal tract. The reported prevalence of CMV retinopathy varies from 4% of ambulatory patients (primarily intravenous drug abusers)11 to 34% of eyes in an autopsy series of male homosexuals.9 This discrepancy may reflect the fact that CMV retinopathy occurs late in the course of AIDS. Most investigators believe that the true prevalence of CMV retinopathy among patients with AIDS is approximately 15% to 25%. It has been suggested that CMV retinopathy is more common in homosexuals, because of different levels of exposure to CMV between risk groups.11,12
Cytomegalovirus retinopathy is clearly a disorder of immunosuppressed persons. Prior to its association with AIDS, the infection in adults was seen almost exclusively in patients with defects in cellular immunity related to disease or immunosuppressive drugs.13,14 A single report of CMV retinopathy in a healthy adult was not confirmed by culture or tissue examination.15
There is no obvious correlation between the development of CMV retinopathy and levels of CMV antibodies. CMV retinopathy has been reported in the absence of detectable antibodies.16
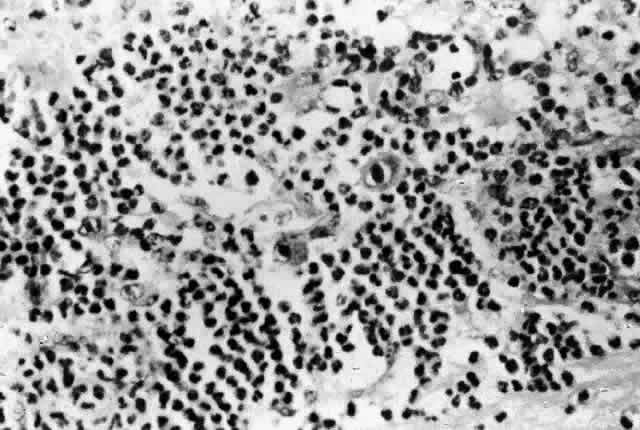
Histologic studies of AIDS-related CMV retinopathy demonstrate the tissue necrosis and cytomegalic cells characteristic of all CMV infections (Fig. 1).9,17 Electron-microscopic and immunochemical studies have identified CMV viral particles and antigen in a patchy distribution throughout all layers of the retina and occasionally in contiguous retinal pigment epithelial cells.9 Viral antigens are only rarely identified in the choroid.9,18 Viral antigen in the choroid has been found in association with vessels and is not always adjacent to areas of retinopathy, suggesting that the virus reaches the choroid through independent hematogenous spread rather than by extension of infection from the retina.
Among cases of AIDS examined at autopsy, 22% to 50% of those with CMV retinopathy have neutrophilic infiltrates in retinal tissue.9,19 This finding is atypical when compared with CMV retinopathy in non-AIDS patients, in which only a sparse lymphocytic infiltrate is observed. The difference has been attributed to intact granulocyte function and chemotaxis in patients with AIDS, in contrast to infants, organ transplant recipients, and patients with malignancies who can have more severe quantitative or qualitative granulocyte dysfunction.9 It has been noted that patients on immunosuppressive drugs can develop marked inflammatory reactions to CMV retinopathy when such drugs are withdrawn.14 Acute inflammatory cells may extend into the adjacent choroid despite the absence of identifiable viral antigens.9 The vitreous usually remains remarkably free of inflammatory material, despite the presence of virus in the vitreous cavity.20
A variety of stimuli (CMV antigen, immune complex deposition, and tissue necrosis) may be responsible for the production of chemotactic factors leading to neutrophilic infiltration. Immunochemical studies have revealed deposition of IgG, IgA, and, to a lesser extent, IgM and C3c in retinal tissue and within retinal arteriolar walls.9 Many IgA-bearing plasma cells were present in one case.17 Perivascular infiltration of neutrophils occurs, consistent with immune complex-mediated vasculitis,9,17 but there is poor correlation between the distribution of tissue-bound immunoglobulins, acute inflammatory cells, and CMV antigens.9 Thus, it is also possible that concurrent retinal infection with other as-yet-unidentified pathogens is responsible for the acute inflammatory reaction seen in these eyes.
Inflammatory cell products do not appear to play a significant role in the extensive tissue destruction common to all cases of CMV retinopathy.
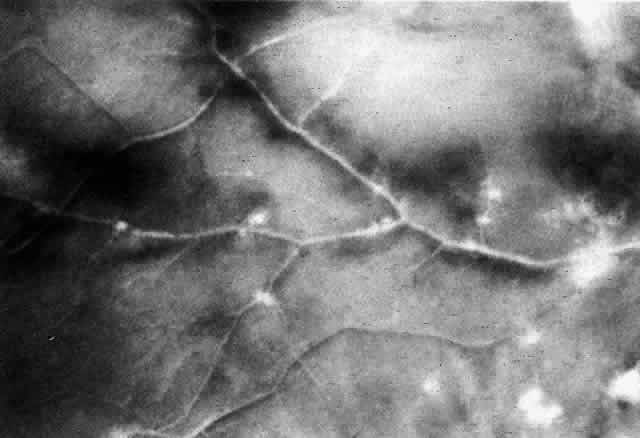
The histopathologic characteristics of AIDS-related CMV retinopathy in one autopsy study are listed in Table 3.9 CMV retinopathy frequently begins adjacent to retinal vessels in the posterior pole (Fig. 2). It is believed that virus reaches the eye via the bloodstream. Untreated CMV retinopathy in patients with AIDS is a relentlessly progressive disease. Usually, infection begins with an isolated focus of disease; rarely are there more than two or three discrete areas of infection within the eye. These lesions invariably enlarge to involve additional retinal tissue. New lesions develop less frequently. The enlargement of lesions does not progress at the same rate from all lesion borders.21 The rate of progression is faster in an anterior direction toward the ora serrata than in a posterior direction toward the fovea. CMV retinopathy has been considered a “foveal-sparing” disease. In a series of organ transplant patients with CMV retinopathy reported by Egbert and associates prior to the AIDS epidemic, only 3 of 21 eyes had macular involvement.14 The higher incidence of macular involvement in early autopsy series of patients with AIDS may reflect the fact that progression of CMV retinopathy into the macular region could not be stopped prior to the introduction of ganciclovir and foscarnet therapy. Even though the fovea can eventually be destroyed, it is usually the last area to become infected; in some cases the disease appears to move circumferentially around the fovea (Fig. 3). Eventually the entire retina will be destroyed, usually within a 6-month period.19 Progression of the retinopathy halts abruptly at the ora serrata.9 Following total retinal necrosis, it is replaced by a thin gliotic membrane.19 No virus can be identified in ocular tissue at this late stage.
TABLE THREE. Autopsy Findings in AIDS Patients: Characteristics of CMV
Retinopathy
| Total patients examined | 35 |
| Cases with CMV retinopathy | 12 (34%) |
| Unilateral | 6 (50%) |
| Bilateral | 6 (50%) |
| Total eyes with CMV retinopathy | 18 |
| Location of retinopathy | |
| Oral | 13 of 18 eyes (72%) |
| Equatorial | 10 of 18 eyes (56%) |
| Posterior | 13 of 18 eyes (72%) |
| Severity of retinopathy | |
| Mild (<10% of retina involved) | 8 of 18 eyes (44%) |
| Moderate (10%–50% of retina involved) | 8 of 18 eyes (44%) |
| Severe (>50% of retina involved) | 2 of 18 eyes (11%) |
| Hemorrhage | 10 of 18 eyes (56%) |
| Acute inflammation | 6 of 12 patients (50%); |
| Choroidal inflammation | 9 of 18 eyes (50%) |
| Serous retinal detachment | 10 of 18 eyes (56%) |
In non-AIDS patients, the spread of disease along vessels has been attributed to infection of vascular endothelial cells, which break free and travel through the retinal circulation.22 In support of this hypothesis, inclusion bodies have been identified in retinal endothelial cells of organ transplant patients with CMV retinopathy. Endothelial cell inclusions have been reported rarely in choroidal tissue of AIDS patients,18,20 but no CMV antigen has been identified in retinal endothelial cells by immunochemical techniques, even in vessels that travel through areas of retinopathy.17 Thus, endothelial cell infection does not appear to play a significant role in the spread of CMV retinopathy in AIDS.
CMV retinopathy is more common among patients with AIDS than among other severely immunosuppressed individuals. The frequent development of CMV retinopathy may be related in part to the retinal microvasculopathy that is common to HIV-infected patients.9,23 Ultrastructural studies of retinal capillaries have demonstrated basal lamina thickening, swelling of endothelial cells, narrowing and occlusion of vascular lumina, and loss of pericytes. This microvascular disease may allow access of virus to retinal tissue through damaged vessel walls. Hematogenous spread of virus to the eye is supported by the finding of CMV-like viral particles within macrophages in the choroidal circulation of an AIDS patient with CMV viremia.19 Sludging of blood flow through the retinal circulation may also contribute to infection by increasing the contact time between circulating infected cells and the retina.24
The only systemic risk factor that has been clearly associated with the development of CMV retinopathy in HIV-infected patients is a low CD4 lymphocyte count.12,25,26 The infection almost always occurs in patients with counts less than 50 per cubic millimeter.
Human cytomegalovirus is highly species-specific, making the study of human CMV infections in animal models difficult. Attention has therefore been focused on murine CMV infections in mice as a possible model for the study of human CMV retinopathy. Holland and associates showed that murine CMV was capable of causing retinal necrosis in immunosuppressed mice.27 Other investigators have subsequently studied the role of immune defenses against murine CMV retinopathy.28,29 There are many differences between murine and human CMV infections of the eye, however, and extrapolations about disease mechanisms to human CMV retinopathy should be made with caution. For example, murine CMV seems to have a propensity for uveal tissue, unlike its human counterpart. Also, susceptibility of murine and human CMV isolates to various antiviral agents is very different, making these models of little use for the investigation of new antiviral agents. Recently, a rabbit model of disease using human CMV was reported.30 Chorioretinal inflammation and destruction are seen, but additional study will be required to determine the extent to which the lesions and the course of disease mimic human retinal infection. The treatment of CMV retinopathy with new antiviral drugs has been reviewed elsewhere.31
Despite its apparent predilection for retinal tissue, CMV is found occasionally on the ocular surface. Cytomegalovirus has been isolated from conjunctival swabs of patients undergoing bone marrow transplantation at UCLA. Also, 19.5% of childhood leukemia victims in one series had CMV in tears.32 The site of infection was not determined in these studies. CMV was found by culture in ocular adnexal tissue of a patient with AIDS and conjunctivitis, but autopsy examination revealed no virus in the lacrimal gland.33 Cytomegalovirus has also been found by electron microscopy in the conjunctiva of an HIV-infected patient without clinical signs of disease related to CMV.34
VARICELLA ZOSTER VIRUS
Zoster ophthalmicus, with cutaneous lesions in the dermatome of the first division of the trigeminal nerve, keratitis, and anterior uveitis, can be seen relatively early in the course of a patient's HIV disease and is believed to be a harbinger of further deterioration in the patient's immune status.35,36 The severity of lesions and inflammation varies widely between patients.
Patients with HIV disease can also develop a chronic, productive varicella zoster virus infection of the corneal epithelium that resembles herpes simplex virus epithelial keratitis.37 This infection has never been reported in immunocompetent patients. It occasionally follows zoster ophthalmicus, with isolation of varicella zoster virus from corneal lesions many weeks after the resolution of cutaneous vesicles. In other patients there is no history of cutaneous zoster.
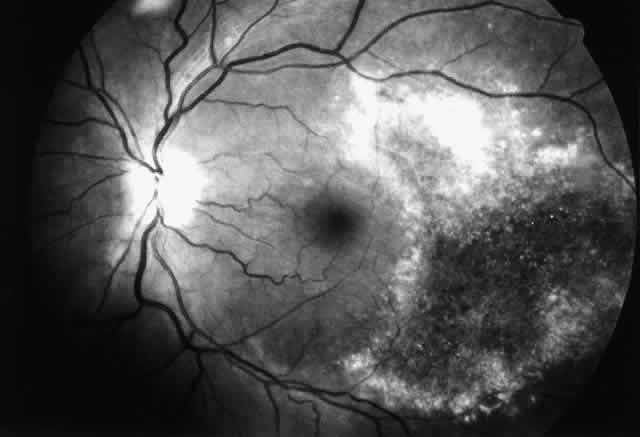
Varicella zoster virus can also cause a severe necrotizing retinopathy in HIV-infected patients (Fig. 4).38–41 It may present as an isolated infection or may occur concurrently with or after cutaneous zoster in any dermatomal distribution. In most HIV-infected patients, varicella zoster virus retinopathy presents as a distinctive clinical disorder known as “the progressive outer retinal necrosis syndrome.”41 It is characterized by multifocal, deep opaque retinal lesions (believed to be retinal edema) that quickly coalesce to involve the entire retina. The retina can become completely necrotic over a period of a few days to a couple of weeks. There is little or no vitreous or anterior chamber inflammatory reaction. Occlusive vasculitis is not a primary feature of the disease, although patients are ultimately left with severe vascular attenuation. The optic nerve may be hyperemic, and optic atrophy is a sequela of the infection.
Histopathologic examination of two cases showed viral antigens and viral particles in the outer retinal layers, consistent with the appearance of deep retinal lesions.39 There were variable amounts of inflammation in the retinas, and scattered areas of choroidal inflammation, although there was no evidence of choroidal infection by the virus.
The infection responds poorly to antiviral therapy.41 Even among patients with response to therapy, recurrence of disease is common.40,41 At the time of disease recurrence, the development of new lesions, including involvement of the second eye in unilateral cases, is more common than reactivation of pre-existing lesions.41 Patients may therefore be having repeated episodes of viremia, or there may be clinically inapparent virus within the retina.
This syndrome is clinically distinct from the classic description of the acute retinal necrosis syndrome, which can also be caused by varicella zoster virus, and is most commonly reported in otherwise healthy individuals. Acute retinal necrosis syndrome is characterized by a prominent vitreous and anterior chamber inflammatory reactions and an occlusive retinal vasculopathy. The difference between the two forms of varicella zoster virus retinopathy cannot be explained solely on the basis of immunodeficiency, because acute retinal necrosis syndrome has been seen in patients with AIDS and other immunodeficiency states.41 The reason that some HIV-infected patients develop acute retinal necrosis syndrome while others develop the progressive outer retinal necrosis syndrome is uncertain. Margolis and associates hypothesized that the clinical presentation of varicella zoster virus retinopathy may be related to the degree of immunosuppression in any given patient.39 The median CD4 lymphocyte count of patients with progressive outer retinal necrosis syndrome is 21 per cubic millimeter.41
Although most reported cases have fallen into one or the other distinct clinical groupings, varicella zoster virus probably causes a spectrum of disease. Patients may have variable amounts of inflammation, lesions may be limited in their distribution, and disease can have variable rates of progression.
HERPES SIMPLEX VIRUS
Herpes simplex virus (HSV) keratitis occurs in HIV-infected patients but may not be more common than infections in the general population. There appear to be differences in epithelial infections between HIV-infected and otherwise healthy patients, however.42 In HIV-infected patients, infection tends to be more prolonged and severe, although most cases will respond eventually to conventional antiviral agents. Infection is more likely to begin adjacent to the corneal limbus. Also, there appears to be a higher subsequent recurrence rate than in healthy individuals. It has been suggested that the infrequency with which stromal keratitis is seen in HIV-infected patients, despite the severity of epithelial disease, is due to the defects in cellular immune function associated with HIV infection.42
Most cases of HSV epithelial keratitis have been caused by HSV type 1 virus, although isolation of both type 1 and type 2 viruses from the same patient has been reported.43 Herpes simplex virus infection of the retina has been reported rarely in patients with AIDS.44,45 In general, HSV retinopathy is a rare disorder and does not appear to be specifically related to immunodeficiency. Most reported cases have had no history of pre-existing immunosuppression. In adults, it is usually seen in association with HSV encephalitis. Pepose and associates reported a patient with AIDS who had concurrent, bilateral CMV and HSV (type 1) infections of the retina as well as HSV encephalitis.44 At autopsy, antigens of both viruses, identified by immunoperoxidase staining, were present in all layers of the retina and in the same geographic distribution. HSV antigen was spread throughout the retina in an even distribution, as opposed to the patchy distribution of CMV antigen. Unlike CMV, HSV could be identified in retinal vascular endothelial cells and in the choroid. Within the brain, HSV antigen was restricted to vascular endothelium and scattered parenchymal cells in the subependymal region only. A similar distribution of virus has been reported by Anderson and Field in an animal model of HSV retinitis using immunocompetent mice.46 In this model, thymidine kinase-negative, acyclovir-resistant viral mutants appeared to have a predilection for retinal tissue. Intracranial inoculation of virus resulted in a severe bilateral necrotizing retinitis yet produced only a mild patchy encephalitis. Anderson and Field suggest that acyclovir treatment may potentially produce viral mutants with increased pathogenicity for the eye. The patient reported by Pepose and associates had been treated with acyclovir, but viral isolates were not obtained from the eye for determination of acyclovir resistance. Schuman and associates described a case of extensive retinal necrosis in which HSV (type 1) antigens were found in the retina at autopsy, but that patient did not have encephalitis.45
It has been suggested that HSV reaches the eye by neural spread from the brain because of the frequent association between HSV retinopathy and HSV encephalitis.47 In the case reported by Pepose and associates, the optic nerves were not infected despite the HSV encephalitis.44 The virus may have reached both the brain and the eye via the bloodstream, as suggested by HSV infection of endothelial cells of the retinal vasculature.
TOXOPLASMA GONDII
Toxoplasma gondii is a common opportunistic pathogen in HIV-infected patients, and toxoplasmosis is believed to be the most common nonviral infection of the brain in patients with AIDS. In contrast, it is a relatively uncommon infection of the eye.
In HIV-infected patients, ocular toxoplasmosis can produce a variety of disorders, including iridocyclitis, retinochoroiditis, and even panophthalmitis and orbital inflammation.48–54 Iridocyclitis is usually secondary to retinal infection and can be severe, resulting in redness, pain, and posterior synechiae formation. In immunocompetent patients, T. gondii infection is restricted to the retina, but infection of the iris has been reported in HIV-infected patients.49
Retinochoroiditis is the most common form of ocular toxoplasmosis. Clinical presentations include single lesions, multifocal lesions in one or both eyes, and broad areas of retinal necrosis.48,55 Small, early lesions may appear to be restricted to the outer or inner retinal layers,48 but in most reported cases, disease has progressed to full-thickness retinal necrosis, usually several disc diameters in size, by the time disease is diagnosed, indicating that untreated disease is continuously progressive. Spontaneous resolution of toxoplasmic retinochoroiditis in HIV-infected patients has not been reported. Progression is generally slow.
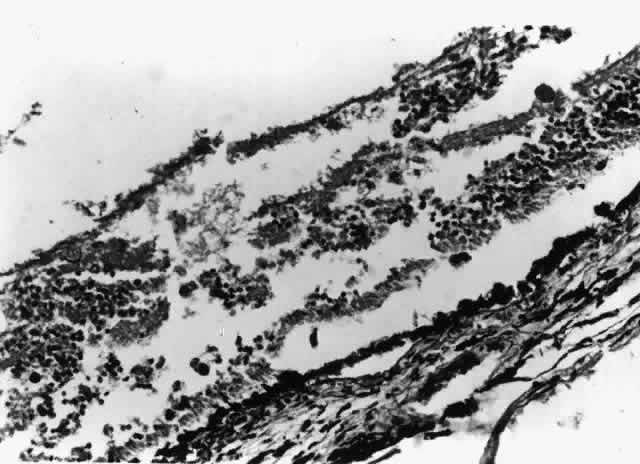
In cases with full-thickness necrosis, the retina appears to have a thick, densely opaque appearance with sharply demarcated borders. There is usually little hemorrhage. There may be inflammatory vascular sheathing, but this sign is not helpful in differentiating ocular toxoplasmosis from other infections, such as CMV retinopathy or syphilis. A prominent inflammatory reaction in the vitreous and anterior chamber has been described by some investigators, while others report little associated vitritis or iridocyclitis.50,51 Histopathologic examination of lesions reveals both trophozoites and cysts in areas of retinal necrosis and within retinal pigment epithelium (RPE) cells (Fig. 5).48 Organisms can be found in fewer numbers in the choroid and vitreous. The optic nerve may also be infected.48,51,52 Although a prominent choroidal inflammatory reaction may be present, there is usually scant inflammatory material in the necrotic retina.48,51
In the general population, most cases of toxoplasmic retinochoroiditis are believed to be due to reactivation of encysted organisms in retinochoroidal scars that are the residua of congenital infections. In HIV-infected patients, lesions are seldom associated with pre-existing scars, suggesting that retinal infection is due to acquired disease or to organisms disseminated to the eye from nonocular sites. The optic nerve has been proposed as one route of spread to the eye, especially in view of the fact that CNS and ocular lesions are commonly seen together. In most cases, however, T. gondii probably reaches the eye via the bloodstream.48
Inactive chorioretinal scars consistent with healed T. gondii infection have been seen in patients with AIDS, but without signs of reactivation.23 Presumably these scars contain viable encysted organisms capable of reactivation. The cellular immunodeficiency of HIV infection may by itself be insufficient to initiate recurrent toxoplasmic retinochoroiditis in previously infected eyes.
Nonhuman primates with healed toxoplasmic retinochoroidal lesions containing encysted organisms do not develop recurrent disease following cellular immunosuppression with total lymphoid irradiation.56 The animals are susceptible to active ocular disease with reinoculation, indicating that cellular immunodeficiency facilitates infection but does not initiate recurrences. The same may be true of the immunodeficiency of HIV infection.
Patients with suspected ocular toxoplasmosis should be evaluated for systemic toxoplasmosis as well. It is believed that 50% of patients with ocular toxoplasmosis also have CNS involvement, and ocular disease may be recognized before any neurologic manifestations of intracranial infection.48,51
Serologic testing is not helpful in diagnosis. Nearly all patients will have anti-Toxoplasma antibodies, but they are also common in the general population, and titers vary widely. IgM antibodies are uncommon.48
Because infection continues to progress without treatment, all cases are treated with antiparasitic agents, although the most effective agents have not been identified. Response to treatment can be remarkably fast.48,51,55 Inflammatory signs resolve without use of corticosteroid therapy. Lesions generally heal completely within 4 to 6 weeks, with little pigmentary response,51 in contrast to the hyperpigmented scars seen in immunocompetent patients. Chronic suppressive therapy must be continued to prevent reactivation of infection.
FUNGAL INFECTIONS
Fungal ulcers of the cornea have been reported to occur without obvious predisposing factors in patients with AIDS.57–59 In otherwise healthy individuals, fungal keratitis usually follows some form of tissue injury or other abnormality of the ocular surface.
A variety of fungi have been reported as causes of retinal or choroidal disease in patients with AIDS (see Table 1), but none are common.45,60–68 They generally are reported to cause multiple, nonspecific lesions in the posterior pole, with variable amounts of inflammation. They are usually late manifestations of AIDS.
Cryptococcus neoformans is the fungus most commonly associated with ocular disease in patients with AIDS. Usually it is reported as a cause of visual loss in patients who have cryptococcal meningitis with damage to the optic nerves or intracranial visual pathways, but intraocular infections have also been reported.60–63 Intraocular organisms have also been found as incidental findings at autopsy,9 where they are seen within retinal and choroidal vessels without associated inflammation. Lack of associated inflammatory reaction is a common finding in many clinical and autopsy studies of cryptococcal intraocular infections in AIDS patients.
Candidal chorioretinitis and endophthalmitis are uncommon in patients with AIDS.62,64 Although mucocutaneous candidal infections are very common in HIV-infected patients, candidal chorioretinitis is not associated specifically with the immunodeficiency state of HIV infection. In a similar manner, patients with chronic mucocutaneous candidiasis, a disease in which there is an isolated specific T-cell defect for Candida sp,69 do not develop candidal chorioretinitis.
Immunodeficiency diseases and immunosuppressive therapies have not been among the common predisposing factors for candidal chorioretinitis in large published series of patients with this infection.70 Among the major risk factors in such patients, however, are intravenous drug abuse and prolonged intravenous catheterization, because they result in candidemia.70,71 The mucocutaneous candidiasis of AIDS does not result in candidemia. Only those patients with AIDS who are active intravenous drug abusers or those who receive intravenous antibiotics or nutritional support through chronic indwelling catheters seem to be at risk for endogenous candidal infections of the eye.
PNEUMOCYSTIS CARINII
Pneumocystis carinii pneumonia is one of the most common manifestations of AIDS. P. carinii can also disseminate to other organs, including the eye, where it can infect the choroid. Choroidal pneumocystosis was first described in HIV-infected patients and is one of the few “new” ophthalmic diseases to have been described as a result of the AIDS epidemic.
For many years, P. carinii has been considered a protozoan parasite, but recent studies have led some investigators to believe that the organism should be classified as a fungus.72–74 Among various pieces of evidence are the facts that it has ribosomal RNA sequences that share greater homology with some fungi than with protozoa, and that it has a soluble translation factor unique to fungal protein synthesis. The issue of taxonomy has not yet been resolved.
Choroidal pneumocystosis is characterized by one or more discrete, yellow-white subretinal plaques in the posterior poles of both eyes. At diagnosis, lesions vary from 300 to 3000 μm in diameter. Lesions are not seen anterior to the equator. Without treatment, choroidal lesions enlarge slowly, with a reported rate of 750 μm/month.75,76 Enlargement may be asymmetric and eccentric, resulting in their sometimes irregular, multilobular shapes. Such enlargement suggests that organisms spread by invasion of choroidal vascular spaces.75 Eventually, large, geographic yellow-white areas result from lesion coalescence. Serous detachment of the retina overlying choroidal lesions has been reported, however.77 There may also be retinal pigment epithelium alterations overlying some lesions.78,79 Histologically, lesions are collections of P. carinii organisms surrounded by a “frothy” material.75,78 There are few or no inflammatory cells in lesions. A vitreous inflammatory reaction is not seen with choroidal pneumocystosis.
Early reports of P. carinii in the retina of patients with cotton-wool spots have never been confirmed in subsequent studies, and there is no evidence that there is any causal relationship between the two.
Many patients with choroidal pneumocystosis have been receiving inhaled pentamidine as prophylaxis against P. carinii pneumonia prior to the development of ocular disease.80 Inhaled pentamidine offers no protection against extrapulmonary P. carinii infections. The discovery of choroidal pneumocystosis after the introduction of prophylactic agents probably reflects the fact that they increase survival sufficiently to allow the emergence of late, uncommon manifestations of the infection. Although choroidal pneumocystosis was not recognized prior to the widespread use of inhaled pentamidine, there are reported cases in patients without a history of prophylactic treatment.75,76
Many patients with choroidal pneumocystosis have concurrent CMV retinopathy. There is a known association between P. carinii and CMV infections in immunosuppressed patients that results in a higher prevalence of concurrent infection of the same organ than would be expected from chance alone.75 In view of the fact that these organisms infect different layers of the eye, their association in AIDS patients may simply be coincidental because of the high prevalence of CMV retinopathy.
Patients with choroidal pneumocystosis are frequently asymptomatic or have only mild visual symptoms, even when lesions are under the fovea.81 The clinical importance of choroidal pneumocystosis is primarily that it can be an early sign of disseminated, life-threatening P. carinii infection. The treatment of pneumocystosis has been reviewed elsewhere.80,81
SYPHILIS
Syphilitic eye disease appears to be more common in HIV-infected patients with syphilis than in others with syphilis. Although ocular disease can be associated with secondary or early latent syphilis, most reported cases in HIV-infected patients have had neurosyphilis.82
Reported ocular manifestations of syphilis in HIV-infected patients have included granulomatous iridocyclitis, vitritis, retinitis, neuroretinitis, and optic neuritis. Syphilitic retinal disease can take a variety of forms in HIV-infected patients. They may have patchy infiltrates, with or without retinal necrosis, that can mimic the more indolent-appearing forms of CMV retinopathy. A very characteristic subretinal lesion has also been described in other patients with HIV infection and syphilis.83 It is typically a large, nonelevated, plaquelike mass found in the macular and juxtapapillary area at the level of the RPE. The borders of the lesion are distinctly yellow, whereas the central area may be more faded. Retinal disease may be unilateral or bilateral. Vascular sheathing may be present.
Syphilitic retinal disease is usually accompanied by marked inflammatory reactions in the vitreous and anterior chamber.82–84 Posterior synechiae are common. Keratic precipitates may be fine white or granulomatous in appearance.
MYCOBACTERIAL INFECTIONS
The AIDS epidemic is responsible in large part for a dramatic increase in the prevalence of tuberculosis over the past decade.85 The cellular immune dysfunction of HIV infection places individuals at increased risk of developing tuberculosis (clinically apparent disease) after infection with Mycobacterium tuberculosis. Intact T-lymphocyte function is necessary to enhance the activity of macrophages that ingest and kill mycobacterial organisms. Extrapulmonary tuberculosis is common in HIV-infected patients and fulfills CDC criteria for the diagnosis of AIDS.
Mycobacterium tuberculosis can infect most tissues of the eye and is a well-known cause of uveitis. Intraocular infection is uncommon, however, even among patients with tuberculosis. There have been only a few isolated case reports of ocular tuberculosis among AIDS patients.86–88 The most common form of ocular tuberculosis is a multifocal choroidopathy. Choroidal tubercules can range in size from less than an optic disc area to several thousand microns in diameter. There can be variable pigmentation; earlier lesions are hypopigmented, with later lesions accumulating some pigmentation. There are no features that are pathognomonic for tuberculosis, and lesions must be differentiated from other mycobacterial infections, P. carinii infection, syphilis, and lymphoma. Choroidal infections can be complicated by scleral extension, serous retinal detachment, and a severe secondary granulomatous anterior uveitis.
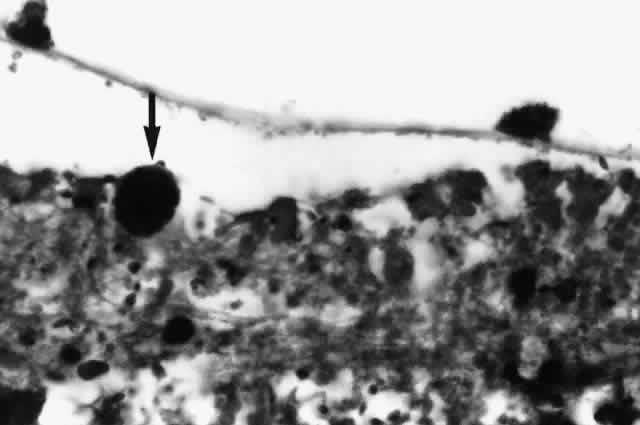
Nontuberculous mycobacterial infections can also produce choroidal granulomata, in which acid-fast bacilli can be found in histiocytes (Fig. 6). Mycobacterium avium complex is the most common cause. Infections have been reported most commonly as incidental findings at autopsy,9,17 although round, yellow-white lesions resembling choroidal pneumocystosis on fundus examination have been confirmed to be nontuberculous mycobacterial granulomata.31
OTHER BACTERIAL INFECTIONS
Bacterial corneal ulcers are uncommon in patients with AIDS.89–92 Infections with Staphylococcus aureus and Pseudomonas aeruginosa have been reported most commonly. They have been related to the same predisposing factors seen in healthy individuals, including epithelial defects, neurotrophic keratitis, and contact lens use. These infections can be severe and difficult to treat, with spread to the sclera and loss of the eye. Metastatic bacterial infections of the retina have been reported rarely.31,93 The focal inflammatory lesions have been nonspecific in appearance and could have been mistaken for a variety of infections.
OTHER CORNEAL INFECTIONS AND EXTERNAL OCULAR DISEASE
Microsporidiosis
The microsporidia are ubiquitous, obligate, intracellular spore-forming protozoan parasites that infect many species. Corneal and conjunctival microsporidiosis is a previously rare disorder that has been reported in several patients with AIDS.94,95 Infection has been caused by members of the genus Encephalitozoon.96It occurs in patients with severe depression of immune function; in one report the mean CD4 lymphocyte count was 26 per cubic millimeter.97
Infection causes a diffuse corneal and conjunctival epitheliopathy. Microsporidial infection of the cornea in patients without HIV infection can result in a corneal inflammatory response. In contrast, corneal microsporidiosis in HIV-infected patients is associated with little inflammation, and the cornea remains relatively clear. The epithelial surface does become very irregular, causing a marked drop in vision and resulting in discomfort. Organisms can be found in the conjunctival epithelium as well, facilitating diagnosis by conjunctival biopsy. Organisms are gram-positive but are seen poorly on hematoxylin and eosin-stained specimens. They have variable staining characteristics with Gomori methenamine silver, acid-fast, and Giemsa stains. A PAS-positive body at one end of the oval mature spore is diagnostic at the light-microscopic level.
The source of ocular infection with microsporidia is unknown. The superficial location of the organisms in the cornea suggests that direct inoculation may be the route of infection. Many of the initially reported patients were owners of pet birds.
Molluscum Contagiosum
Molluscum contagiosum is the most common infection of the eyelids in HIV-infected patients.98–100 The responsible DNA virus is ubiquitous throughout the world and commonly infects healthy adults and children, causing small nodular lesions on the skin. In the general population, lesions involving the eyelid margins characteristically cause a chronic follicular conjunctivitis. HIV-infected patients tend to have more lesions, and they are much larger; it is common to see numerous lesions covering the face and eyelids. Even large eyelid margin lesions do not tend to cause follicular conjunctivitis, however, probably because of the patients' suppressed cellular immunity. Lesions are usually tolerated well, but very large, bulky lesions or lesions involving the inner portion of the eyelid margin may be uncomfortable; these lesions will shrink with cryotherapy, but it is difficult to eradicate lesions completely.
The AIDS epidemic is providing researchers with an unprecedented opportunity to investigate the interaction between immune defense mechanisms and pathogenic organisms in a variety of settings, including the eye. As more is learned about the complex functioning of the immune system and the specific defects in AIDS, insight can be obtained into ways in which the body protects the eye against microbial disease.