The papillomavirus virion consists of an outer protein capsid, 55 nm in diameter, enclosing the DNA. The capsid is made of 72 capsomeres that form an icosahedron. The capsid is composed of two groups of proteins: a major protein (molecular weight [MW] 54 kd) and a minor protein (MW 76 kd). The major protein is antigenically similar between animals and humans. The minor protein appears to be type-specific. Papillomavirus coats differ from those of other DNA viruses in that they do not have an outer lipid envelope. They are resistant to inactivation by freezing, ether, and desiccation, but sensitive to high temperature, aldehyde fixatives, and detergents.
Classification of papillomaviruses is based on the host organism and further typed and subtyped in accordance with their degree of genomic homology. A virus is named on the basis of its natural animal host, and host specificity is high. Using solution hybridization, viral isolates demonstrating less than 50% homology are considered new types, isolates demonstrating more than 50% are considered subtypes, and those with almost 100% homology are considered variants. It is important to realize that 50% hybridization corresponds to almost 90% homology of DNA sequence.
Antigenic serotyping of papillomaviruses as is done with adenoviruses is not possible because of insufficient antigen and the failure of the virus to replicate in vitro. To date, more than 70 types of HPV have been identified, and these have been divided into the following three groups:
Genital-mucosal lesions
Nongenital lesions
Epidermodysplasia verruciformis4
The types most often seen in the periocular tissues are from the first group.
Another method of grouping HPV subtypes is on the basis of clinical behavior, as follows:
High-risk subtype: Those associated with invasive malignant tumors (e.g., HPV-16, HPV-18)
Low-risk subtype: Those associated with benign tumors (e.g., HPV-6, HPV-11).
Simultaneous infection with multiple HPV subtypes has been reported.
The HPV genome is a closed circle of double-stranded DNA, 7900 base pairs in length. The genome consists of nine open reading frames (ORFs) and an upper regulatory region (URR), all located on one strand of the DNA.5 The nine ORFs encode nine proteins, which are divided into seven early or “E” proteins and two late or “L” proteins, which are capsid proteins. El ORF encodes proteins that are important in modulating DNA replication.6,7 E2 ORF encodes proteins that regulate viral gene transcription.8–10 E4 ORF encodes a protein that makes up a large portion of wart tissue but whose function is unknown.11 E5 ORF encodes a small protein found in the cell membrane that can transform cultured murine cells.12 E6 ORF encodes a protein involved in cellular transformation and control of the number of viral DNA copies. E7 ORF encodes a protein involved in transcriptional transactivation and cellular immortalization.
PATHOGENESIS AND TUMORIGENESIS
HPV infection is thought to occur when virus is inoculated into viable epithelium. Loss of integrity of epithelium is thought to enhance susceptibility to infection.13 The viral DNA infects basal cells of the epithelium. There it remains extrachromosomal and replicates along with the host cell DNA.14 Viral DNA episomes are carried upward through the differentiating epithelium. In the malpighian cell layer, transcription of viral DNA occurs. Viral DNA replication occurs in the granular cell layer, and hundreds of viral DNA replicas may be made. The viral capsid proteins are then synthesized and the virions assembled in the host cell nucleus as it progresses to the stratum corneum.
Specific questions regarding HPV tumorigenesis arise: Why do certain types cause certain pathology? Are there group differences between high- and low-risk types?
It appears that HPV DNA in benign lesions from low-risk types rarely inserts in the host genome but exists as an episome, whereas in severe dysplasia and carcinoma associated with high-risk HPV types, the HPV DNA incorporates into the host genome.15 Moreover, neoplasia is not simply the result of viral DNA insertion or its site of insertion, but of insertion of the viral DNA into the host genome with concurrent disruption of the E2 ORF. Disruption of the E2 ORF allows for increased production of E6 and E7 proteins, which appear to exert important effects on the tumor suppressor proteins p53 and the product of the retinoblastoma (Rb) gene.
Tumor suppressor proteins typically have regulatory functions that affect normal cell growth. Homozygous absence of these proteins allows loss of normal cell regulation and tumorigenesis, as in the example of the Rb gene where absence of a normal copy of the Rb gene leads to clinical retinoblastoma. E6 binds and accelerates the degradation of p53 protein, which is active in limiting cell reproduction.16 E6 protein from high-risk HPV-16 and HPV-18 is more effective at inactivating p53 than that from low-risk types. High-risk E6 binds E6-associated protein, which is necessary for the degradation of p53; low-risk E6 binds p53 but does not lead to degradation.17,18 High-risk HPV DNA also encodes truncated E6* proteins, whose function is not yet known.15
E7 protein binds the product of the Rb and p107 genes, both of which are negative growth regulators.19,20 Although originally thought to require E6 to cause immortalization of cells, E7 can immortalize cells by itself when in the presence of a strong promoter.21,22 However, it does appear that E6 and E7 act synergistically in the tumorigenic process.
In studies of HPV infection of cultured cells, so-called cytopathic effects—loss of contact inhibition, alteration of cell shape, loss of growth factor dependence, and immortalization—have been described. Three of the early genes, E5, E6, and E7, are believed responsible for these properties. The amounts and forms of these proteins produced vary with different HPV subtypes and often correlate with their clinical behavior.
ROLE OF HUMAN PAPILLOMAVIRUS IN PERIOCULAR DISEASE
The relationship between periocular squamous epithelial proliferations and viral infection was historically addressed by the categorization of these lesions as viral (infectious) and noninfectious.23 Viral papillomas were believed to occur more often in young patients and to be essentially benign in nature. Noninfectious papillomas were thought to be related to ultraviolet exposure, more commonly found in adults, and capable of undergoing malignant transformation. The role of HPV was explored because of similarities in the histology of HPV-induced cervical disease.
The earliest work by Lass and associates24 using immunohistochemical techniques directed at antigens of the viral capsid demonstrated HPV involvement in 2 of 21 conjunctival papillomas examined. In a subsequent immunohistochemical study, McDonnell and colleagues25 detected HPV-common antigen in 46% of 50 papillomas and 8% of 61 dysplastic or carcinomatous lesions of conjunctival epithelium. Subsequent application of more sensitive DNA in situ hybridization techniques showed HPV-11 DNA in one case in which HPV antigen was detected.26 Other cases of papilloma with detectable HPV antigen and DNA were reported.27–29 Using in situ DNA hybridization, HPV 6 or HPV 11 was detected in 65% of 23 papillomas but in none of 28 dysplastic lesions.30 In a study using polymerase chain reaction (PCR)-aided analysis, six of six tissue specimens with conjunctival or corneal dysplasia were found to have HPV-16 DNA, but not HPV-18 DNA.31 Lauer and co-workers used PCR-aided analysis to detect the E6 region of HPV DNA in four of five conjunctival intraepithelial neoplasia lesions.32 HPV-16 was found in all four lesions, and coinfection with HPV-18 was found in two of these. One study from an experienced laboratory did not detect HPV-6, -11, -16, or -18 in specimens of squamous cell carcinoma of the conjunctiva with the use of either in situ hybridization or PCR-aided analysis, and the authors speculated that another HPV type might be involved.33 In a larger series, HPV-16 DNA was found using PCR-aided analysis in 88% of 42 tissue specimens and in 83% of six conjunctival swabs from patients with dysplastic disease.34,35 A small series of bilateral squamous conjunctival tumors associated with HPV-16 also has been reported.36
Of the eyes in the McDonnell PCR DNA studies35,36 in which conjunctival swab specimens were found adequate for analysis, the clinically uninvolved eye of one patient showed evidence of HPV DNA and HPV-16 DNA that persisted years after “successful” excision of the lesion in the affected eye. This finding demonstrates the following: (1) an unsuspected subclinical HPV infection can exist; and (2) induction of clinical disease might be multifactorial.30,34 In the McDonnell studies,35,36 corneal swabs from women with cervical HPV-16 disease contained HPV-16 DNA in 76.5% of patients, none of whom had clinical ocular disease.
Data on HPV detection in other squamoproliferative conditions have been limited. Identification of HPV in pterygia has been controversial. One study using immunohistochemical analysis of HPV antigen found HPV in 56% of pterygia patients and in 25% of controls.37 Other studies have used pterygia as negative control tissue in which HPV DNA was not detected.30,34 HPV-16 DNA has been detected in recurrent squamous cell carcinoma of the eyelid.38 HPV DNA also has been detected in epithelial tumors of the lacrimal sac.39 Three of three papillomas contained HPV-11 DNA. One of three carcinomas studied contained HPV-18 DNA. In another study,40 a case of inverted papilloma was positive for HPV antigen and polytypic DNA probe, whereas specific probes for HPV-6 and HPV-11 were negative.
In summary, HPV-6, -11, -16, and -18 have been identified in periocular squamous epithelial lesions. Similar to findings in the anogenital region, HPV-6 and HPV-11 are associated with benign lesions and HPV-16 and HPV-18 with dysplastic or malignant lesions. Infection can be found in the following cases: (1) in the clinically normal eyes of patients with infection of the other eye and after successful excision of lesions; and (2) in the clinically uninvolved eyes of patients with cervical infection. This may reflect the multifactorial nature of this disease. The rates of HPV detection in all types of ocular disease approach those in anogenital disease when PCR-aided analysis is used.
EPIDEMIOLOGY
The analysis of HPV-related lesions of the periocular tissues is complicated by a variety of factors, including the advent of new technologies, demographic considerations, occult infections, immune deficiency, and risk factors such as solar exposure and smoking. As mentioned above, some types of lesions now known to harbor HPV in at least some cases were previously not thought to be virus induced. Thus both the spectrum and prevalence of HPV-related periocular disease have been underestimated. Demography is an important issue because these lesions are relatively uncommon, and each has a distinct clinical and pathologic presentation, affecting very different age groups and both genders. Until extensive studies are done regarding the prevalence of HPV infection in these diseases, a comprehensive discussion of ocular HPV demographics will not be possible. Demographic factors can be considered both by studying the demographics of HPV infection in other sites where data are available and by analyzing demographic characteristics based on the clinical type of lesion.
General Demographics
The only reported systematic demographic study of ocular disease (thought to have an HPV-related etiology) has evaluated the occurrence of histologically proved conjunctival dysplasia and carcinoma during a 10-year period in Brisbane, Australia.41 An average incidence of 1.9 cases per 100,000 persons per year was calculated. The male to female ratio was 2.3:1.
WOMEN. The most comprehensive available demographic data have been reported on anogenital lesions, specifically those of the female genital tract. The incidence of new anogenital condylomatous lesions or dysplasias in the United States is 1 million per year.42 Since infection can exist without clinical disease, however, the incidence of infection is probably much higher. This is evident from the data on anogenital HPV infections. Among female patients aged 14 to 23 years old, 32% had cervical swabs that contained HPV DNA.43 In two thirds of these specimens, HPV-16, HPV-18, or HPV-31, types frequently associated with cervical carcinoma, were detected. Among 505 Norwegian women requesting first-trimester termination of conception, 6.1% had detectable HPV in cervical swabs.44 In a study of older women (65 and older), 3.5% had HPV DNA detected in cervical swabs.45
OCCULT INFECTION AND IMMUNE DEFICIENCY. Among men, occult HPV infection has been found in up to 40% of genitourinary clinic patients with the use of cytology and DNA hybridization studies.46 Homosexual men can have up to a 65% prevalence of detectable HPV in their anal mucosa.47 The fact that occult infection with HPV-16 was found in the oral mucosa of 43% of 62 asymptomatic subjects without significant known risk factors is perhaps an even more significant finding.48
Immune deficiency enhances the manifestation of HPV clinical disease. Warts were seen in 43% of unselected renal transplant patients.49 An increased incidence of skin lesions also was seen in a group of AIDS patients.50
OTHER RISK FACTORS. Other factors associated with squamoproliferative diseases include solar exposure and smoking. A case control study in the ophthalmic literature claimed there was no effect of solar exposure on squamous epithelial disease.51 A larger study in a renal transplant population, however, showed that warts and carcinomas were more prevalent in this group compared to control subjects. When patients were separated on the basis of high versus low solar exposure, all carcinomas and more than twice the incidence of warts were found in the group with high solar exposure.49
Ocular Transmission
Ocular transmissibility of HPV is inferred from the fact that sexual partners of patients with genital HPV have an increased rate of infection and that neonates of mothers with genital HPV may become ocularly infected at birth.52,53 An infant of a mother with condylomata acuminata was found to have conjunctival and respiratory papillomas that tested positive for HPV-6 DNA.54
DEMOGRAPHICS. Demographic data are available on the clinical periocular lesions likely to be related to HPV infection or transformation. These include eyelid warts, eyelid papillomas, conjunctival papillomas, conjunctival dysplasia and carcinoma, lacrimal sac papillomas, and pterygia.
Nonocular cutaneous HPV lesions most commonly occur in patients between the ages of 12 to 16 years old, but demographic data on clinically diagnosed eyelid papillomas are not available. In the slightly older sexually active population, anogenital lesions are becoming more common. Conjunctival papillomas comprised 12% of epibulbar lesions; 7% of these occurred in patients who were less than 20 years old.55 In a later series of 302 epibulbar lesions in children less than 17 years old, conjunctival papillomas constituted 7% of lesions.56 Conjunctival papillomas are more often multiple in children. Despite the large number of patients with AIDS who show multiple molluscum contagiosum of the eyelids and an increase in nonocular papillomas, a similar increase in HPVassociated lesions in the conjunctiva or eyelids has not been noted in this group.
Conjunctival intraepithelial neoplasia and squamous cell carcinoma are most common in late adulthood, with a 3:1 to 20:1 male to female ratio. Patients with squamous cell carcinoma are an average of 10 years older (mean = 68.7 years; range = 41 to 96 years) than patients with dysplasia (mean = 58 years; range = 8 to 90 years).57,58 Conjunctival intraepithelial neoplasia and squamous cell carcinoma are thought to be associated with solar exposure, although collecting accurate direct quantitative data of ocular sun exposure has proved difficult.
CLINICAL PRESENTATION AND DIAGNOSIS
Comprehensive discussions of the presentation and diagnosis of each lesion presumed to be HPV-induced are beyond the scope of this chapter and have been reviewed elsewhere.59,60 Rather, a brief discussion of each lesion type will be presented.
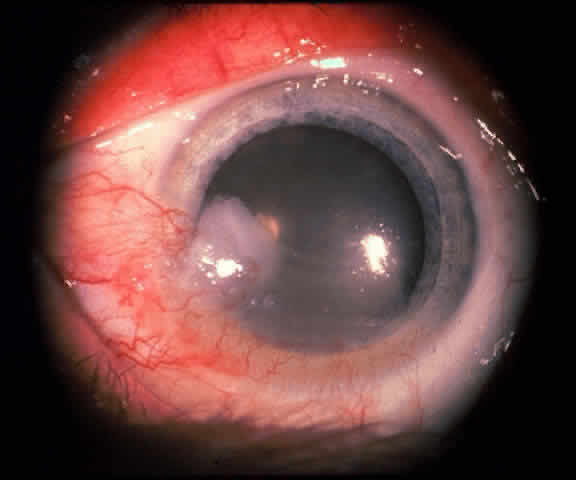
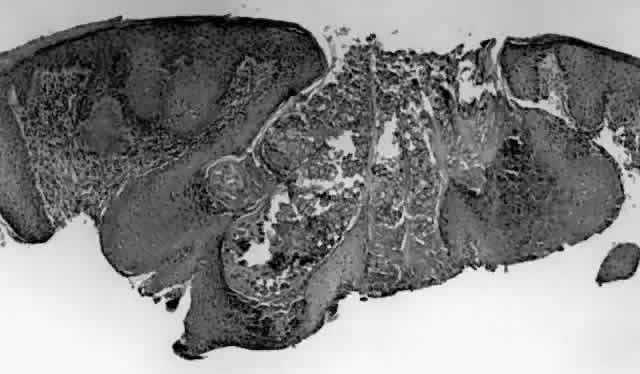
In the conjunctiva, HPV can induce papillomatous lesions (papillomas and inverted papillomas) and epithelial dysplastic lesions consisting of conjunctival intraepithelial neoplasia and squamous cell carcinoma. Conjunctival papillomas were traditionally divided into infectious, limbal, and inverted types; however, current data suggest that all types have a viral etiology. All sites of the conjunctiva can be affected, and involvement of the contiguous epithelium of the lacrimal canaliculus, lacrimal sac, and nasolacrimal duct has been noted to occur.38,61,62 Conjunctival papillomas can be pedunculated or sessile and are typically shiny, flesh-colored, and papillomatous (Fig. 1). Each papillary frond has a vascular core that is easily seen through the nonkeratinized epithelium. The papillomatosis is responsible for the typical appearance of evenly spaced vessels throughout the lesion. If keratinized, these lesions may appear whitish and dull. Papillomas can be either unilateral or bilateral, and they can be either solitary or multifocal (Fig. 2). Inverted papillomas are the least common type of papilloma in all sites, but they constitute a larger portion of lacrimal sac tumors than other periocular tumors.63,64
|
|
The diagnosis is usually made on the basis of the history and clinical appearance. In questionable cases, excisional biopsy should be performed. Conjunctival papillomatous lesions must be differentiated from squamous cell carcinomas, which may occur as a papillomatous variant indistinguishable from both typical papillomas and other squamoproliferative lesions.
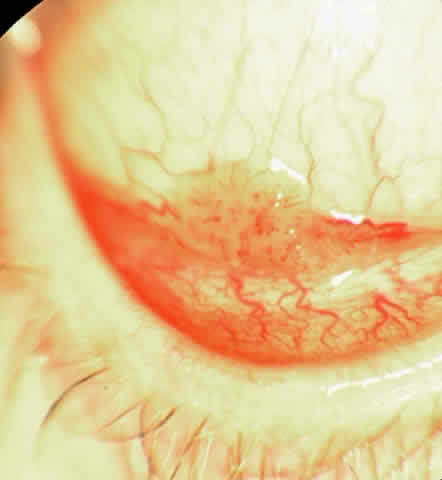
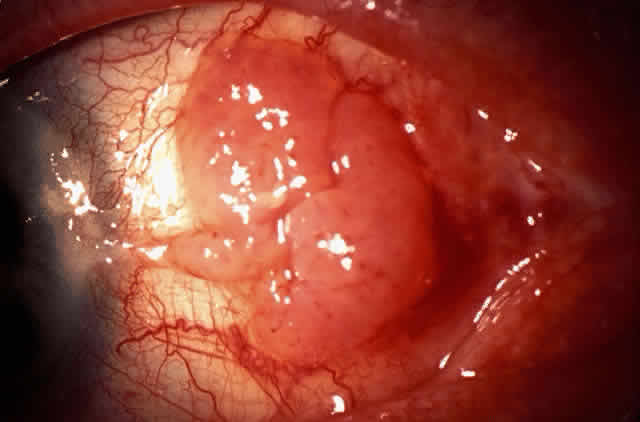
The epithelial dysplastic lesions—conjunctival intraepithelial neoplasia and squamous cell carcinoma—almost always begin at the limbus, most commonly in the interpalpebral zone.57,58 The initial appearance is a gelatinous, gray lesion with some thickening (Fig. 3). There may be increased vascularization. The epithelial changes may extend onto the cornea, causing a hazy, gray appearance. Tumor vascularity lags behind the lead margin of the epithelial change. As the lesions progress from dysplastic to carcinomatous, they become thicker, more vascularized and may show keratinization, giving a leukoplakic appearance (Fig. 4). Lesions confined to the epithelium move freely over the sclera, whereas invasive lesions are fixed to underlying tissue. Occasionally squamous cell carcinomas appear identical to classic papillomas.
|
|
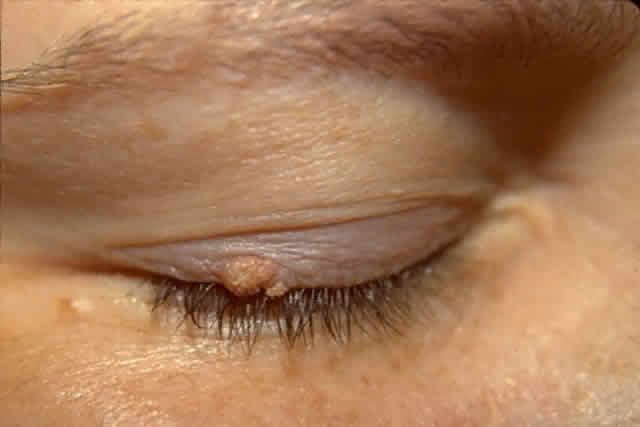
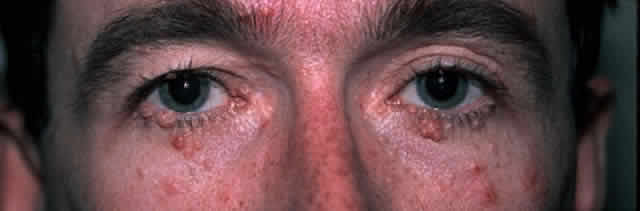
The diagnosis is at best suspected clinically but histologic confirmation allows a distinction between epithelial dysplasia and entities such as pterygium, keratosis, melanoma, nevus, and other lesions. Pterygia are almost always in the horizontal meridian. A lesion centered away from the limbus and one in other than a horizontal meridian should be suspect for a dysplastic lesion. Handling and processing these specimens to ensure that an accurate diagnosis can be made requires the active participation of a pathologist with experience evaluating this type of specimen. Eyelid papillomas have the typical appearance of skin warts with a flesh colored, papillomatous surface (Fig. 5). Hyperkeratotic scaling is common. Dysplastic and squamous cell carcinomas of the eyelids typically show an elevated flesh colored lesion with hyperkeratosis and abnormal vasculature. Eyelid lesions must be differentiated from seborrheic keratosis, acrochordons, nevi, and other squamoproliferative lesions. Lacrimal sac papillomas and carcinomas are very uncommon tumors.63 Patients present with epiphora and a medial canthus mass, usually above the medial canthal tendon. Bloody epiphora is suggestive of malignancy. Diagnosis is made with the use of imaging and biopsy.
|
PATHOLOGY
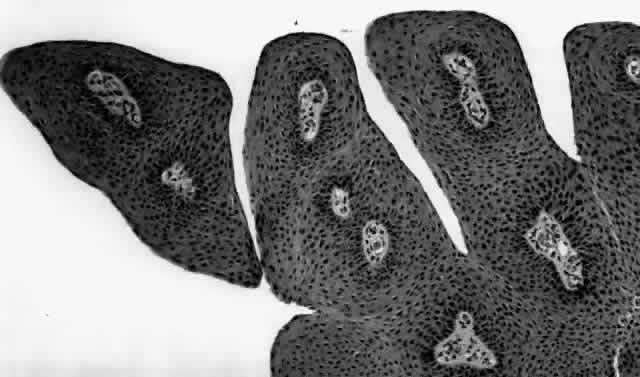
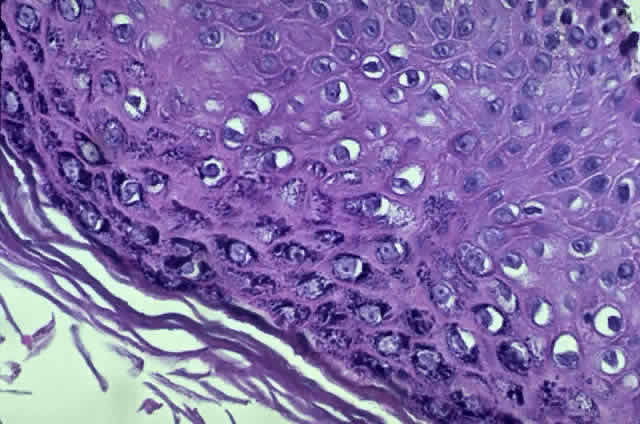
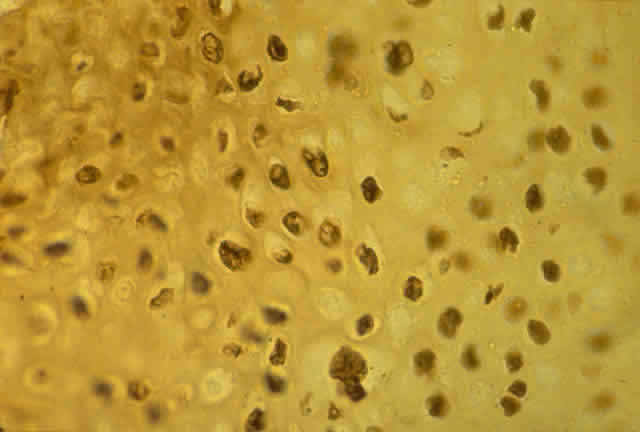
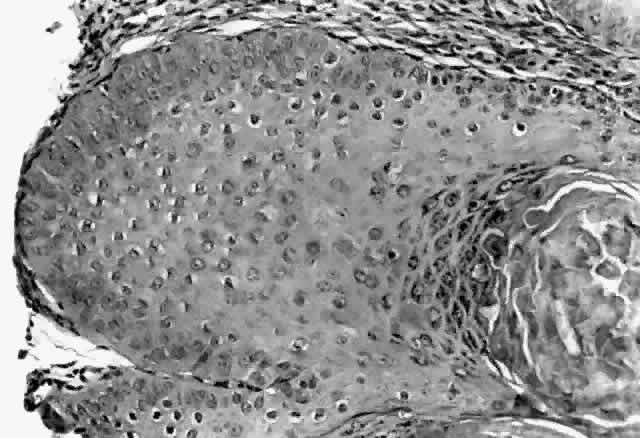
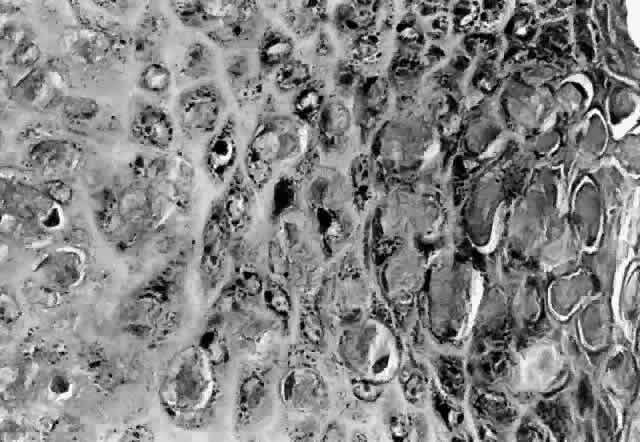
HPV-induced conjunctival squamous papillomas demonstrate papillomatosis, acanthosis, and koilocytosis (Fig. 6). Because they are mucous membrane lesions, those of the conjunctiva and lacrimal sac show no granular cell layer but will show keratinization if they are large or exposed (Fig. 7). In our experience the frequency of koilocytes is variable, even in lesions almost certain to be viral (Fig. 8). Scattered inflammatory cells may be present in the cores or epithelium. Virus can be detected immunohistochemically in the nuclei of the cells (Fig. 9).
|
|
Early dysplastic lesions (conjunctival intraepithelial neoplasia) show thickened epithelium with extension of basal-like cells beyond the basal layer, but cells continue to mature into flat squamous cells. Mitoses are also seen higher in the epithelium. As the degree of dysplasia worsens, the more superficial epithelium is replaced at higher levels by basal-type cells, mitoses can be seen further from the basal layer, and atypia of cells becomes more pronounced. With squamous cell carcinoma in situ, there is full-thickness dysplasia, cellular atypia is variable, and mitoses can be seen at all layers of the epithelium. Invasive squamous cell carcinomas are usually well differentiated. Pleomorphism, hyperchromaticity, dyskeratosis, and horn pearls are seen. Epithelial cell clusters can be seen invading below the basement membrane. Inflammatory cells are common in the substantia propria.
Squamous papillomas of the eyelid are exophytic lesions with multiple fronds, each showing acanthotic epithelium around a fibrovascular core. There is typically hyperkeratosis and koilocytosis. Inflammatory cells may be present.
The histopathology of lacrimal sac epithelial tumors is typical of papillomas or squamous cell carcinoma. Koilocytosis may be present.
Historically, viral involvement was indicated by the presence of koilocytosis. Later, immunohistochemical analysis of HPV-common antigens allowed the first demonstration of viral components in lesional cells (see Fig. 8). Newer, more specific DNA-based techniques allowed typing of viruses. In situ hybridization, which bridged DNA and immunohistochemical techniques, was somewhat more sensitive than either technique alone. The development of PCR amplification allowed detection of a tiny amount of original source DNA and, with proper controls, powerful statements could be made about specific virus involvement. Because DNA is relatively stable compared to many antigens, DNA techniques can be used on archival material or on specimens fixed with aldehydes and embedded in paraffin.
TREATMENT AND PROGNOSIS
The treatment of periocular HPV-induced lesions is divided on the basis of anatomic considerations. In contrast to the epithelium of the eyelid skin and genital tract, where tissue destruction can be better tolerated, the ocular surface has a delicate balance of tear film and epithelial and stromal tissues, and therefore treatment must be gentler to afford preservation of vision. Certain HPV treatment modalities thus are not applicable to periocular lesions. Additionally, most treatment data were generated before the full spectrum of HPV involvement in periocular epithelial disease was suspected and before the biology of HPV infection was understood, and thus the theoretic rationale behind these original approaches is of uncertain value.
Excision or local destruction of involved tissue has been the mainstay of HPV-induced lesion treatment. When the distinction between infectious or viral papillomas and other presumably noninfectious lesions was made on clinical grounds, excisional surgery was deemed adequate for noninfectious lesions, whereas infectious lesions required some attempt to “sterilize” the base of the lesion. In view of our current understanding, such distinctions are probably of little value. Modalities that have been used to achieve sterilization include cryotherapy, cautery, chemical agents, keratolysis, and radiation.57,58,65,66
Special surgical modalities such as the argon and carbon dioxide laser have been used as to destroy periocular HPV lesions. While initially reported to be more effective than routine surgery in cervical lesions, further studies showed no difference between the two.67,68 In addition, the plume of vaporized tissue has been shown to contain HPV DNA, which could theoretically cause spread of disease, specifically of the respiratory tract, to operating room personnel and the surgeon.69,70 Such cases have not been reported, and one study has suggested that the HPV material recovered from the plume is not clinically infectious.71 This issue is still not fully resolved, and the fact that many patients have simultaneous infection with HIV and other viruses warrants care before selecting vaporizing modalities and employment of proper plume-management techniques.
Chemotherapeutic efforts have involved antimetabolites. Topical 5-fluorouracil has appeared effective in treating lesions of the female genital tract. The sensitivity of the ocular surface, however, is an important consideration; topical 5% 5-fluorouracil applied to the rabbit cornea was found to retard epithelial healing.72 This agent and the related agent mitomycin C might have the potential to retard epithelial proliferation without affecting the virus specifically. These agents can cause serious complications, including severe corneal scarring and melting of the ocular connective tissue coat. Recent studies have claimed no significant side effects associated with topical 0.01% mitomycin C solution when used for pterygium or conjunctival intraepithelial neoplasia.73,74
Immunotherapeutic efforts have employed dinitrochlorobenzene and interferon. Isolated case reports using multiple topical applications of dinitrochlorobenzene after systemic sensitization have been successful.75–77 Intralesional injection was used to augment the effect. The method has not been studied in any series or compared to alternate methods, leaving its efficacy uncertain. Interferon alfa has been used in the treatment of recurrent conjunctival papillomas following reports of initial success in other sites.78 Patients in the initial report of five cases underwent surgical excision of the tumor, followed by daily intramuscular injections of 5 million units interferon alfa-n1 for 1 month.79 This interferon was extracted from cultured lymphoblastoid cells. Administration was then decreased to two to three injections weekly for 6 months. Subsequently, some patients had administration stopped, while others continued on more prolonged therapy. All patients experienced reduction in the rate of tumor recurrence and extent of disease; however, three patients experienced recurrence, in two of whom the severity of the disease was judged to be less after interferon treatment. An additional case of interferon treatment of papillomas has also been reported.80
Other factors must be considered regarding the use of interferon. Systemic side effects can be significant, including fever, chills, weight loss, leukopenia, thrombocytopenia, and alterations in renal and hepatic function.81,82 Side effects have occurred with recombinant interferon alfa-2b as well as with extracted human interferon.83 Intralesional injections of cervical lesions still resulted in side effects in 83% of patients. Long-term studies of laryngeal papillomatosis and cervical disease have shown no long-term benefit compared to other modalities.84 In addition, interferons are extremely expensive, and the treatment regimen is very demanding. Currently, condyloma acuminata of the anogenital region is the only indication for interferon use in HPV disease that is approved by the Food and Drug Administration.
Although treatment of HPV-induced disease has been challenging, other agents might provide benefit. Retinoic acid (vitamin A) has been shown to regulate squamous epithelial differentiation. It inhibits growth of HPV-18-positive immortalized cervical carcinoma cells and downregulates production of proteins E6 and E7 in HPV-16-infected human keratinocytes.85,86 Because HPV replication is dependent on the state of differentiation of the epithelial cells, retinoic acid might alter HPV replication. Retinoic acid can also prevent malignant transformation. Retinoids have been initially effective in treating warts in immunosuppressed patients, but lesions have recurred after cessation of therapy. Topical retinoic acid has been investigated for the treatment of keratoconjunctivitis sicca and appears to be noninjurious to the ocular surface.87
Treatment of conjunctival papillomas remains enigmatic, with recurrence rates approaching 40% or more. Ironically, the dysplastic HPV-induced lesions, conjunctival intraepithelial neoplasia, and squamous cell carcinoma have higher rates of successful treatment. Recurrence of conjunctival intraepithelial neoplasia or squamous cell carcinoma is less than 5% when excision and cryotherapy are used together, compared to 33% when either modality is used alone.66 It is hoped that a better understanding of the pathogenesis of these lesions will allow for a more efficacious treatment.
Skin carcinoma and dysplasia are effectively cured with excision and frozen-section control of the margins. Metastasis occurs less than 1% of the time.
The number of lacrimal sac lesions treated has been relatively few, and management consists primarily of wide local surgical excision. Adjunct radiation therapy can be effective in some cases.