|
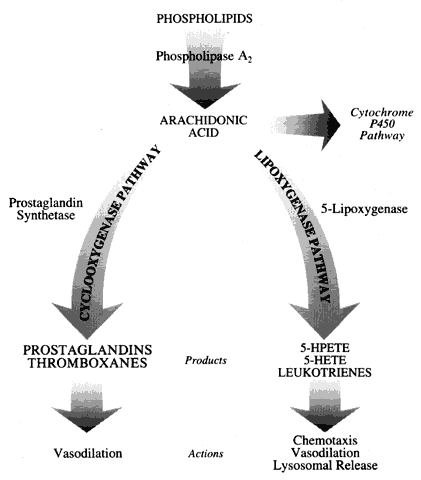
Initial studies into immunogenic models of keratitis provided encouraging results. Belfort and co-workers62 found that indomethacin, a cyclooxygenase inhibitor, was capable of decreasing the clinical inflammatory response to intracorneal injection of bovine gamma globulin. Despite this decrease in inflammation, there were no statistically significant differences in immune response. Antibody titers in the aqueous, vitreous, and serum were very similar in both the indomethacin-treated eyes and the untreated eyes. Antibody production by cells in the uveal tissues and homolateral lymph nodes was also similar between the two groups.
Verbey and colleagues63 examined the effect of suprofen (a cyclooxygenase inhibitor), Rev 5901 (a lipoxygenase inhibitor), and Bay 08276 (both a lipoxygenase inhibitor and a mild cyclooxygenase inhibitor) in an immunogenic keratitis model utilizing intracorneal injection of human serum albumin. All animals were examined by slit lamp and pachymetry. All untreated animals developed corneal edema and clouding, Wessely's ring formation, and neovascularization. All of these changes resolved after 30 days. Treatment with 0.1% fluorometholone (a corticosteroid) prevented all of these inflammatory and neovascular signs. Treatment with Rev 5901 resulted in a decrease in the period of corneal opacification, less intense opacification, and less intense Wessely's ring formation. Neovascularization was significantly decreased. Bay 08276 treatment resulted in a slightly greater inhibition in adverse clinical findings compared with Rev 5901, but these differences were not statistically significant. Treatment with suprofen decreased the intensity of corneal edema, but not the duration of corneal opacification. The duration of the neovascular response was shortened significantly. Combination treatment with suprofen and Bay 08276 produced the same changes in clinical course as produced by Bay 08276 treatment alone (shorter duration of corneal opacification and neovascularization and inhibition of corneal edema compared with controls).
Verbey and associates63 concluded that cyclooxygenase inhibition does not affect leukocyte infiltration and neovascularization, but does decrease corneal edema through its action on prostaglandin formation. The efficiency of leukotriene B4 inhibition by lipoxygenase inhibitors is proposed as the mechanism for inhibition of inflammation. These authors did not present data on the inhibition of these products in the same animal model; however, they did calculate the inhibitory effects based on the work of other authors who used whole-cell assays. Clinical corneal opacification that was not associated with thickening of the cornea by pachymetry was interpreted as leukocyte infiltration; however, no histologic evaluation or radiolabeling of leukocytes was used to evaluate the actual number of inflammatory cells present.
Leibowitz and co-workers21 evaluated suprofen's ability to suppress inflammation in an animal model of chemical keratitis using clove oil. They measured inflammation using radioactively labeled leukocytes. They found that if suprofen was initiated at the same time or 24 hours after induction of inflammation, there was no effect on corneal inflammation. However, if pretreatment with suprofen was begun 48 hours before clove oil injection and continued after injection, there was a significant decrease in leukocyte infiltration into the cornea. In the setting of a clinical infection, obviously pretreatment with an anti-inflammatory agent is not possible.
Other authors have investigated the effect of NSAIDs in animal models of infectious keratitis.30,33,64 Gritz and associates33 investigated the course of infectious keratitis in a rabbit model treated with vehicle, prednisolone, and flurbiprofen (a cyclooxygenase inhibitor). In the absence of antibiotic therapy for Pseudomonas keratitis, there was a more rapid progression of disease in the prednisolone-treated animals and further progression with flurbiprofen treatment. The progression to descemetocele was more rapid for the flurbiprofen group when compared with either the vehicle- or prednisolone-treated groups (p < 0.01). With antibiotic treatment for Pseudomonas keratitis, there were no differences among the groups in regard to clinical examination or antibiotic action as measured by residual viable bacteria after 24 hours of antibiotic treatment. Infectious keratitis secondary to Streptococcus pneumoniae did not demonstrate a clinical worsening when treated with anti-inflammatory agents in the absence of appropriate antibiotic therapy. There were no differences among the three groups in terms of the number of residual viable bacteria after 24 hours of antibiotic therapy.
Ohadi and colleagues30 investigated the effects of prednisolone, flurbiprofen, nordihydroguariatic acid (NGDA; a lipoxygenase inhibitor), and SKF104353 (a lipoxygenase antagonist) in a guinea pig model of Pseudomonas keratitis. No statistical differences were seen in the progression of clinical disease in the absence of antibiotic therapy in this model. Flurbiprofen-treated animals did tend to have a larger area of corneal involvement compared with the control group (p = 0.22). Concomitant treatment with both an antibiotic and each of the anti-inflammatory agents did not demonstrate any differences in the clearing of bacteria as measured by quantitative culture. NDGA-treated animals exhibited greater conjunctival swelling compared with the other treatment groups. This difference was statistically significant between the NDGA- versus the prednisolone-treated animals (p < 0.05). This difference could have been due to NGDA toxicity or the effects of NDGA on the inflammatory and disease processes. Conjunctival discharge was significantly decreased in the flurbiprofen-treated group, compared with the animals treated with either vehicle or one of the leukotriene antagonists (p < 0.01). No differences were seen among the groups in terms of resultant corneal scarring. Small sample sizes relative to the standard deviation of the measurements were a problem with this study.
Hobden and co-workers64 stratified rabbits by age in their investigation of flurbiprofen and ciprofloxacin in the treatment of Pseudomonas keratitis. Animals were divided into three treatment groups and received saline, 0.3% ciprofloxacin, or a combination of 0.3% ciprofloxacin, 1% prednisolone acetate, and 0.03% flurbiprofen. They began treatment of the animals at a very early stage in the model, before significant stromal edema or infiltrate occurred. Slit-lamp grading of inflammation showed significantly less inflammation among animals in the same age group treated with flurbiprofen; however, there was no statistical difference in this group in comparison with the other groups in terms of the number of polymorphonuclear leukocytes present, as measured by myeloperoxidase activity. Younger animals had significantly worse inflammation and more leukocyte infiltration compared with older animals in the same treatment group.
The animals in this study had a much milder keratitis than those in previous studies. In a model like this, some animals may not develop infectious keratitis clinically, despite intrastromal injection of bacteria.1,33 Because the only treatment group that received anti-inflammatory treatment received a combination of corticosteroids and a cyclooxygenase inhibitor, it is impossible to differentiate between the individual effects caused by each of these anti-inflammatory medications.
The effect of flurbiprofen on epithelial HSV keratitis was investigated by Trousdale and co-workers.65 Animals were treated with placebo, 0.1% dexamethasone, or 0.1% flurbiprofen. Treatment with either the corticosteroid or the cyclooxygenase inhibitor caused a significant worsening in corneal opacity, area of corneal involvement, and conjunctivitis compared with the placebo group. There was no difference in the degree of worsening between the corticosteroid and the cyclooxygenase inhibitor. No antiviral medications were available at that time, and thus these animals did not receive antiviral treatment.
Hendricks and colleagues66 investigated the effect of flurbiprofen on an animal model of stromal HSV keratitis. Animals were treated with dexamethasone or flurbiprofen and no antiviral agent. Dexamethasone had a variable effect, ranging from significant improvement in the degree of inflammation to significant exacerbation of disease. Flurbiprofen did not exacerbate disease in any of the animals and resulted in reduction or no change in the stromal opacity. In vitro studies in cell culture demonstrated a virucidal effect for flurbiprofen, but none for dexamethasone. The authors suggested that the improved stromal opacity could be due to the virucidal effect. There is a lack of consistent results in this animal model, and the results of this study therefore should be viewed with this fact in mind.
There have been fewer investigations into the use of NSAIDs compared with corticosteroids in the treatment of ocular infections. Additional agents in the NSAID class are being developed. There is a possibility of increased morbidity when these agents are used in the absence of appropriate antimicrobial therapy. Therefore, the guidelines that we recommend for NSAID use are similar to those given for steroid use in ulcerative keratitis (see above). From the studies performed thus far, this class of therapeutics does not seem to have any advantages over corticosteroids in regard to decreasing inflammation or subsequent corneal scar formation. In fact, the cyclooxygenase inhibitors may increase leukocyte infiltration when used after initiation of inflammatory insult and thus may be detrimental.
CYCLOSPORIN A
Cyclosporin A (CsA) is a immunosuppressant with very specific, targeted actions focusing around the basic events surrounding T-cell lymphocyte activation. Normally the activation of noncommitted, resting T-cells requires both specific and nonspecific signals.67 Activation of the T-cells leads to expression of receptors on the cell membrane of the T-cell, including the receptor for the lymphokine interleukin 2 (IL-2). This receptor is believed to be the primary mechanism for recruitment and activation of new T-cells.68 CsA appears to block the formation of IL-2 receptors, the stimulation of IL-2, and its ongoing production. There is conflicting evidence as to the population of T-cells that are affected by CsA.68 There is general agreement that there is prevention of induction of cytotoxic T-cells, but the effect on suppressor T-cells is less clear. This subset of cells may be spared from suppression or may be induced by CsA administration, depending on the investigation and the animal model used for investigation.
Because of the mechanism of action of CsA, the applications most extensively investigated regard control and prevention of corneal graft rejection. Systemic and topical administration has been investigated regarding corneal graft rejection, intermediate and posterior uveitis, and noninfectious, immune-related corneal ulcers.68–73 Animal models have shown topical CsA to be of use in corneal graft rejection, but adequate placebo-controlled studies in humans are lacking. A large study regarding systemic CsA in high-risk patients is underway. Certain uveitis patients appear to achieve better control when systemic CsA is used. In human case series, marginal corneal ulceration related to autoimmune disease can be improved through the use of topical and systemic CsA.69–72
No investigations have been published regarding the use of CsA in immunomodulation therapy in infectious ocular disease. CsA has been shown to worsen the course of herpetic keratitis in the absence of antiviral therapy. CsA could possibly be used in the therapy of stromal HSV keratitis. Patients who experience a steroid-responsive glaucoma constitute one group that could benefit from CsA therapy. No studies have been done to investigate this potential use. A multicenter study would be required to recruit adequate numbers of patients to address this question properly. In light of the Herpetic Eye Disease Study, it would be more difficult to design an ethical study to evaluate the use of CsA for stromal HSV keratitis. Because of its effect on cell-mediated immunity, CsA could be of use in the control of inflammation seen with Toxoplasma infection of the eye. These studies also have yet to be performed.
INTERFERON
Interferons are a group of glycoproteins secreted by mammalian cells in response to viral and parasitic infections and other stimuli. This multifunctional group is part of the host's defenses against viral and parasitic infections and certain tumors.74 Through induction and synthesis of a number of proteins, they exert a number of effects on the immune system and also affect cell proliferation and differentiation. Interferons are classified according to the animal of origin (e.g., human, murine) and the antigenic specificities (designated alpha, beta, and gamma). Biosynthetic interferon and interferon purified from cell preparations have been found to have similar clinical antiviral activity.75 The different subtypes of interferon have been shown to have different levels of activity, both in vitro and in vivo.
Most of the investigations into ophthalmic applications of interferon have dealt with viral infection, mainly HSV. Smolin75 reported an improvement in stromal and epithelial HSV keratitis with topical or intramuscular interferon therapy compared with placebo in an animal model. This improvement was observed with interferon treatment alone (no concomitant treatment with antiviral agents), regardless of whether therapy was begun before or up to 2 days after inoculation with HSV. In additional experiments, Smolin reported that combined treatment with acyclovir and interferon was significantly better than either therapy alone.
Neumann-Haefelin and associates76 used an African green monkey model to investigate the effect of interferon therapy in HSV keratitis. They found that prophylactic and simultaneous administration of interferon resulted in inhibition of keratitis. When interferon was administered after inoculation with virus, only a slight moderation was observed in the course of keratitis.
Three studies have reported human trials of combined therapy with topical acyclovir and alpha interferon for dendritic HSV keratitis.77–79 Colin and co-workers78 used “human leukocyte interferon,” but there was no further description of the source (human cells or recombinant DNA) or interferon subtype. Subjects in the study received 3% acyclovir ointment five times per day and either interferon or placebo once per day. Both treatment groups were comparable at the initiation of treatment. Interferon treatment resulted in a much faster healing of the corneal epithelium over the ulcer. De Koning and co-workers77 performed a similar trial using topical acyclovir and alpha interferon derived from human leukocytes. The groups were similar before therapy was begun and, once again, more rapid healing was observed with interferon treatment. In both studies, the time needed for epithelial healing was almost twice as fast in the treated group when compared with control patients.43
Sundmacher and associates79 examined recombinant human interferon alpha and gamma. They reproduced the same degree of improved epithelial healing when the same concentrations of either type of interferon (30 million IU/mL) were used alone. When interferon alpha and gamma were used in combination, however, a very low concentration (1.5 million IU/mL) of each type produced the same degree of improvement. Similarly low concentrations of a preparation containing only one type of either interferon did not produce more rapid healing.
An interesting case of topical recombinant interferon alpha-2a treatment for HSV keratitis was reported, in which ulcerative HSV keratitis developed in a renal transplant patient who was on chronic immunosuppressive and acyclovir treatment.80 The HSV strain was resistant to multiple antiviral agents, both in vitro and in vivo. After 7 weeks of multiple therapies, including trifluridine, vidarabine, debridement, and topical and systemic acyclovir, interferon was added to topical acyclovir therapy. The keratitis quickly resolved. Moderate epithelial toxicity was noted after 11 weeks of therapy, and interferon and acyclovir were discontinued. A dendritic ulcer recurred 5 weeks later. Topical antiviral agents were, once again, ineffective alone and the addition of topical interferon resulted in resolution of the keratitis.
Interferon therapy has been reported to be of help in patients with another viral-related ocular disease, hepatitis C.81,82 Three patients with chronic hepatitis C infection presented with severe corneal ulceration resembling Mooren's ulcer, which are painful, crescentic peripheral corneal ulcers.81–83 Cultures of these ulcers were negative for bacteria, fungi, and viruses. Despite treatment with topical antibiotics, topical and systemic corticosteroids, NSAIDs, and topical cyclosporine drops, the ulcers continued to progress. Because these patients had a positive serology for hepatitis C, they were given systemic interferon alpha-2b treatment, which has been shown to be effective in suppressing the associated hepatic inflammation. All three patients improved with this therapy, and each received 6 months of continued interferon therapy. All patients were doing well at 1 year of follow-up.
Clinical investigations into the use of interferon therapy for adenoviral conjunctivitis have been less promising. In two clinical trials of interferon alpha-2, there were no significant effects on the duration of clinical disease, virus shedding, occurrence of bilateral infection, and subepithelial infiltrates.83,84 Adams and co-workers83 found that, although there was no difference in the incidence of bilateral infection between the control and treatment groups, symptoms in the second eye were less severe in the interferon-treated group. Unfortunately, both of these studies involved relatively few patients. Despite the negative findings of these two studies, a larger scale study involving more patients may reveal advantages of topical interferon in patients with adenoviral keratoconjunctivitis.
Interferon alpha has been shown to inhibit endothelial cell migration and proliferation. Because of these properties, it has been successfully used to treat pediatric pulmonary hemangioma and hairy cell leukemia. When topical and systemic interferon was used in an animal model of corneal neovascularization, however, no inhibition in corneal vascular growth was observed.85 Topical prednisolone acetate did inhibit neovascularization in this study, but no additive effect was seen when interferon was added to prednisolone treatment. This study used silk sutures to induce corneal neovascularization. It is possible that the mechanisms inducing neovascularization after infection-related inflammation may be different than is present in this model. It is also possible that other dosages of interferon may effect corneal neovascularization.