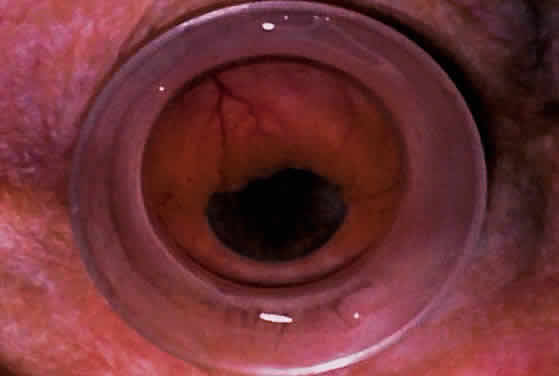
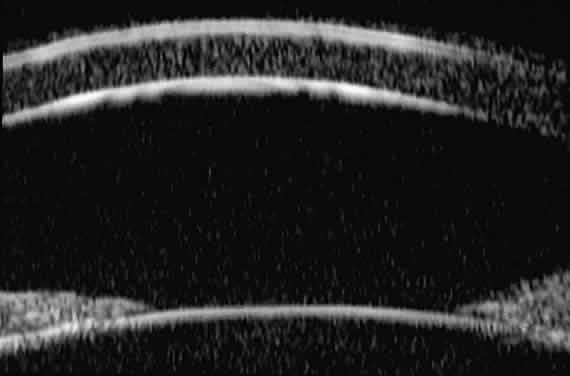
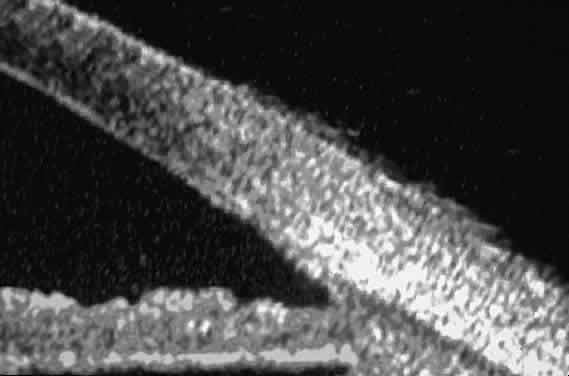
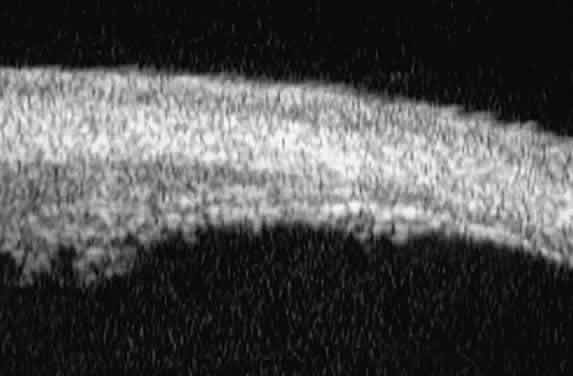
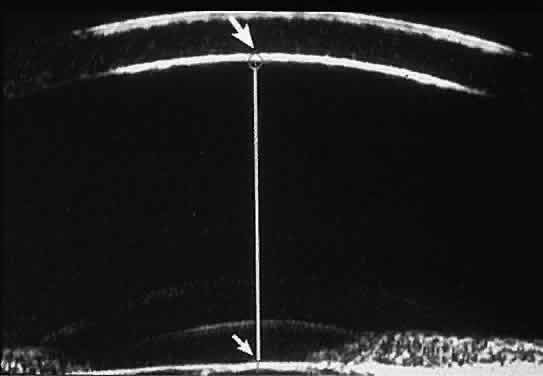
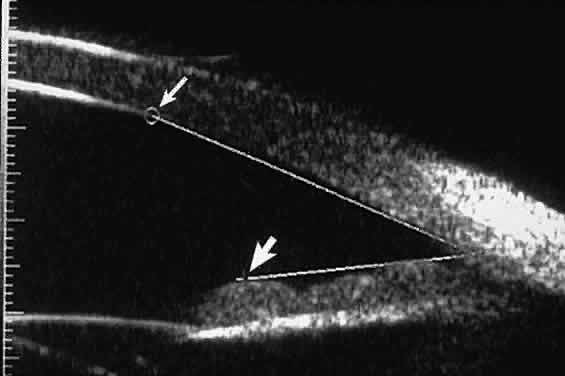
| Ultrasound biomicroscopy can be used to image various structural abnormalities
and lesions of the anterior segment.2–4 It has been used most extensively in glaucoma but has also contributed
greatly to our current understanding of the various disorders and lesions
of the cornea, iris, lens, and ciliary body. GLAUCOMA Ultrasound biomicroscopy is usually able to determine the mechanism of
elevated intraocular pressure (angle closure versus open angle) by showing
the relationship between the peripheral iris and the trabecular meshwork.2–4 A strong correlation between gonioscopic and UBM estimates of angle configuration
has been established.5 In addition, imaging of the anterior segment structures is possible even
in eyes with profound corneal edema that precludes gonioscopic assessment
of the angle. In open-angle glaucoma, UBM can be used to measure the anterior chamber
angle in degrees, to assess the configuration of the peripheral iris, and
to evaluate the trabecular meshwork (Fig. 9).2,4 The angle configuration can be graded and compared with gonioscopic findings. In
certain patients with open-angle glaucoma, UBM can provide
information that may be of some diagnostic value (Fig. 10). For example, in pigment dispersion syndrome (see Fig. 10A),6 UBM typically reveals posterior bowing of the peripheral iris (“q” configuration
of peripheral iris by Spaeth classification5). In plateau iris syndrome (see Fig. 10B),7 UBM usually reveals abnormally steep anterior angulation of the peripheral
iris (“s”configuration of peripheral iris by Spaeth classification5), insertion of the iris from the anterior ciliary body, and retroiridic
projection of the ciliary processes. In eyes with peripheral anterior
synechiae (see Fig. 10C and D), UBM can reveal the extent of iridocorneal adhesion even if the cornea
is hazy or opaque. 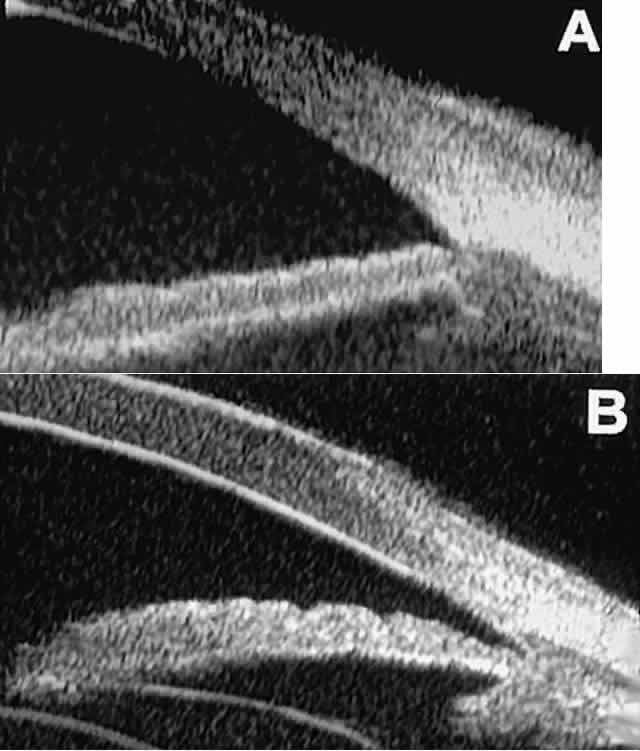 Fig. 9. Angle configuration in eyes with open-angle glaucoma. A. Wide open angle with flat iris plane (D40r configuration by Spaeth gonioscopic
grading system). B. Moderately wide angle with anteriorly bowed iris plane (C30r by Spaeth
gonioscopic grading system). Fig. 9. Angle configuration in eyes with open-angle glaucoma. A. Wide open angle with flat iris plane (D40r configuration by Spaeth gonioscopic
grading system). B. Moderately wide angle with anteriorly bowed iris plane (C30r by Spaeth
gonioscopic grading system).
|
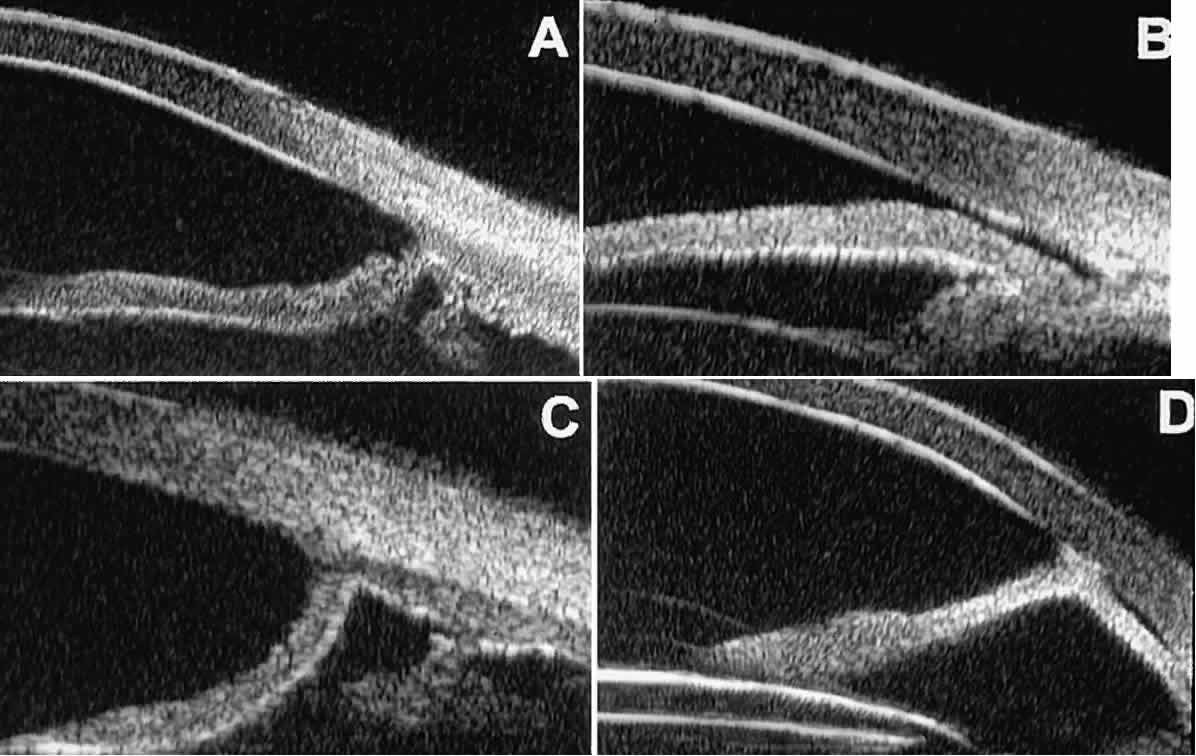 Fig. 10. UBM features of special glaucoma cases. A. Pigment dispersion syndrome with posterior bowing of peripheral iris (“q” configuration by Spaeth gonioscopic grading system). B. Plateau iris syndrome with origin of iris from anterior surface of ciliary
processes behind peripheral iris, slitlike narrowing of peripheral
angle, and abrupt transition from steep peripheral iris to flat iris
midzone. C. Broad peripheral anterior synechia with posterior bowing of nonadherent
iris. D. Peripheral anterior synechia with aqueous-filled slit between site of
iridocorneal adhesion and iris root after cataract extraction with implantation
of posterior-chamber IOL. Fig. 10. UBM features of special glaucoma cases. A. Pigment dispersion syndrome with posterior bowing of peripheral iris (“q” configuration by Spaeth gonioscopic grading system). B. Plateau iris syndrome with origin of iris from anterior surface of ciliary
processes behind peripheral iris, slitlike narrowing of peripheral
angle, and abrupt transition from steep peripheral iris to flat iris
midzone. C. Broad peripheral anterior synechia with posterior bowing of nonadherent
iris. D. Peripheral anterior synechia with aqueous-filled slit between site of
iridocorneal adhesion and iris root after cataract extraction with implantation
of posterior-chamber IOL.
|
In eyes with a narrow angle, UBM shows the extent of angle closure, reveals
the depth of the anterior and posterior chambers, and identifies
pathologic processes pushing the lens and iris forward (Fig. 11).2–4,8 UBM has been able to differentiate between primary angle closure (i.e., cases of angle closure without additional pathology responsible for the
anterior lens-iris displacement [see Fig. 11A] and secondary angle closure due to processes such as lens swelling
and dislocation (see Fig. 11B), massive hemorrhagic retinal detachment pushing the lens and iris anteriorly (see Fig. 11C), and multiple neuroepithelial cysts of the iridociliary sulcus (see Fig. 11D). 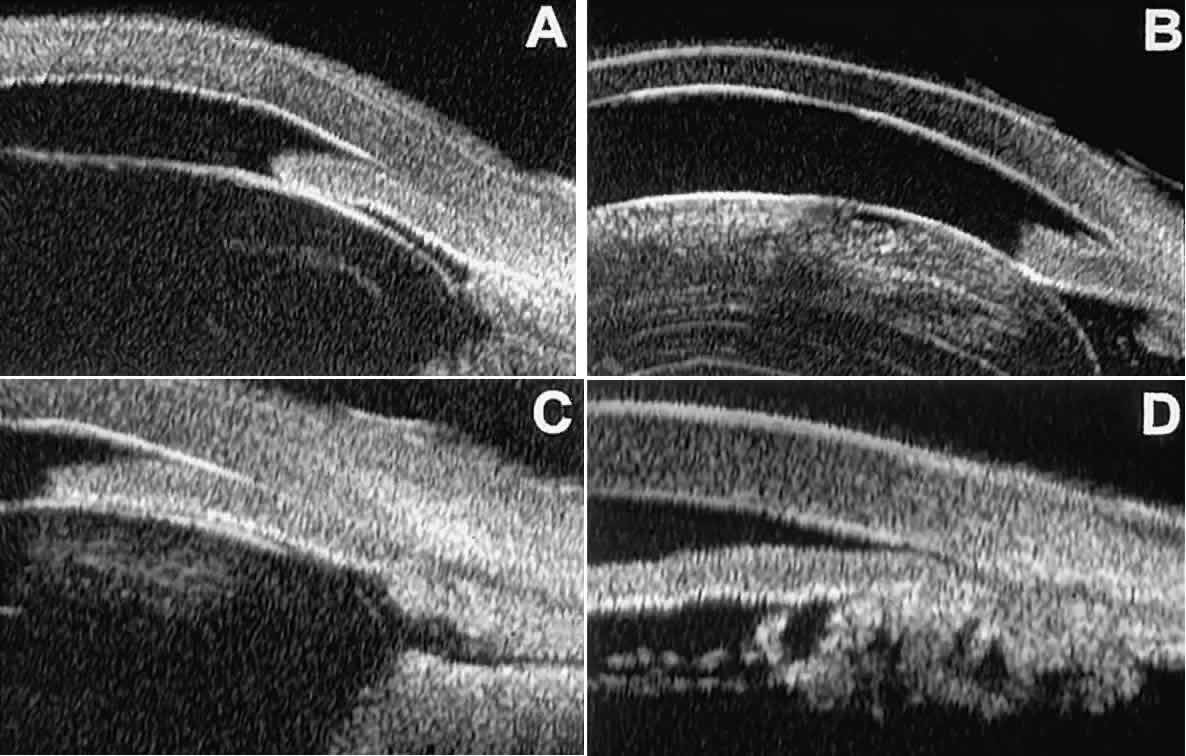 Fig. 11. Angle configuration in eyes with angle-closure glaucoma. A. Primary angle-closure glaucoma with anterior displacement of lens and
iris. B. Angle closure secondary to swollen, cataractous lens (phakomorphic angle
closure). C. Angle closure secondary to massive hemorrhagic retinal detachment; the
subretinal blood is evident in the lower right corner of the photograph. D. Angle closure secondary to multiple peripheral iris cysts. Fig. 11. Angle configuration in eyes with angle-closure glaucoma. A. Primary angle-closure glaucoma with anterior displacement of lens and
iris. B. Angle closure secondary to swollen, cataractous lens (phakomorphic angle
closure). C. Angle closure secondary to massive hemorrhagic retinal detachment; the
subretinal blood is evident in the lower right corner of the photograph. D. Angle closure secondary to multiple peripheral iris cysts.
|
Postoperative UBM imaging of the anatomic changes caused by glaucoma surgery
often helps to explain mechanisms of success and failure of the
various surgical procedures (Fig. 12).3,4 After laser iridotomy, UBM can show whether the iridotomy is partial thickness (see Fig. 12A) or full thickness (see Fig. 12B) and whether the plane of curvature of the peripheral iris has changed
compared with the pretreatment findings. After trabeculectomy (see Fig. 12C), UBM can show whether the scleral aperture is patent or blocked internally, whether
the peripheral iridectomy is open or blocked, and whether
the filtering bleb is flat, shallow, or deep.9 After tube shunt surgery (see Fig. 12D), UBM can show the position of the tip of the tube and whether its orifice
is open or plugged. 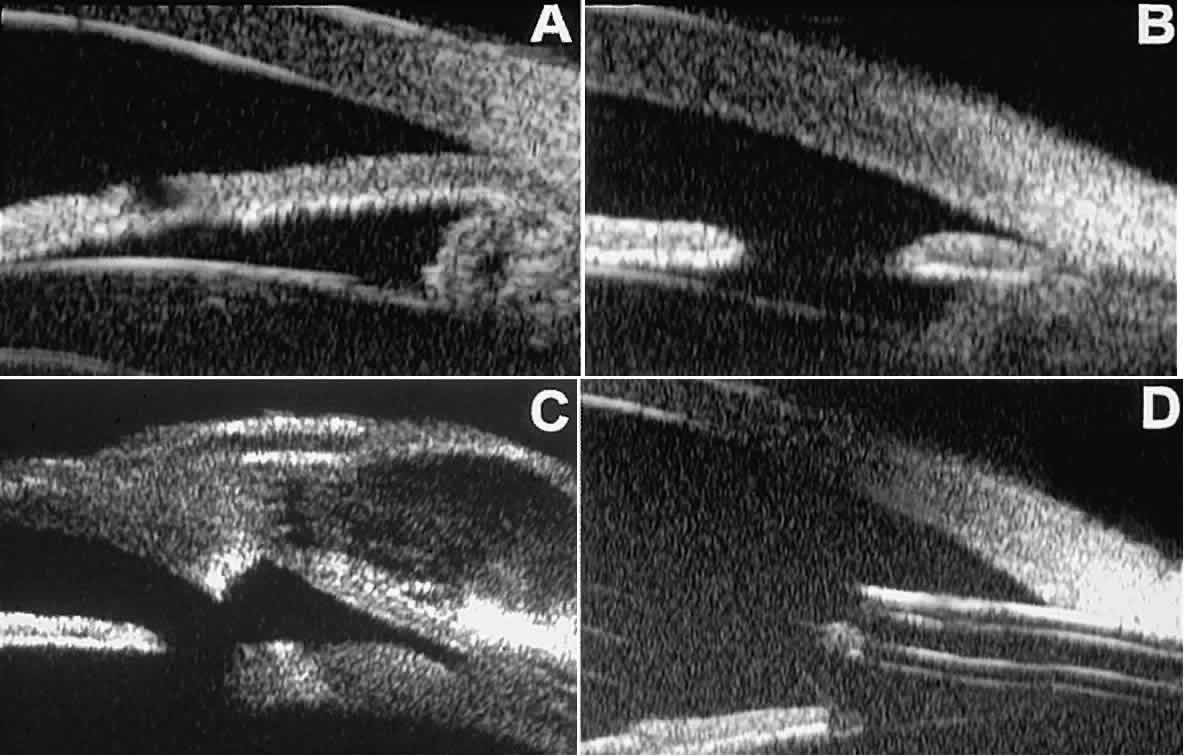 Fig. 12. UBM features in glaucomatous eyes after treatment or filtering surgery. A. Incomplete peripheral iridectomy created by laser. B. Full-thickness peripheral iridectomy created by laser. C. Postoperative features of trabeculectomy including peripheral iridectomy, inner
scleral defect, thin residual scleral flap, and overlying conjunctival
filtering bleb. D. Tube shunt projecting radially into anterior chamber; note that the tube “shadows” deeper structures. Fig. 12. UBM features in glaucomatous eyes after treatment or filtering surgery. A. Incomplete peripheral iridectomy created by laser. B. Full-thickness peripheral iridectomy created by laser. C. Postoperative features of trabeculectomy including peripheral iridectomy, inner
scleral defect, thin residual scleral flap, and overlying conjunctival
filtering bleb. D. Tube shunt projecting radially into anterior chamber; note that the tube “shadows” deeper structures.
|
After any type of glaucoma filtering surgery,10 UBM can be used to detect and evaluate the extent of postoperative complications
such as ciliochoroidal effusion and cyclodialysis.3,4 In ciliochoroidal effusion (Fig. 13A), UBM shows the ciliary body to be edematous and separated from the sclera
by a sonolucent collection of supraciliary fluid. Many ciliochoroidal
effusions that are too limited in extent to be detectable by indirect
ophthalmoscopy and slit lamp biomicroscopy can be imaged by UBM. In
cyclodialysis (see Fig. 13B), UBM shows a well-defined separation between the uveal tissue and the
sclera in the region of the scleral spur. The width of the cleft is usually
assessed best by means of limbus-concentric images through the
region of interest. 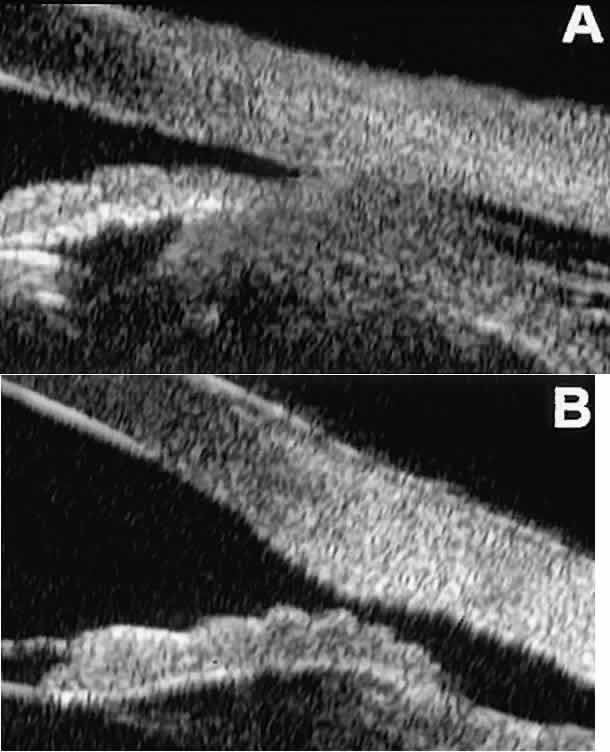 Fig. 13. Complications of intraocular surgery. A. Postoperative ciliochoroidal effusion appears as slitlike spaces filled
with serous fluid posterior to scleral spur. B. Postoperative cyclodialysis appears as complete separation of iris and
ciliary body from sclera in region of scleral spur. Fig. 13. Complications of intraocular surgery. A. Postoperative ciliochoroidal effusion appears as slitlike spaces filled
with serous fluid posterior to scleral spur. B. Postoperative cyclodialysis appears as complete separation of iris and
ciliary body from sclera in region of scleral spur.
|
Ultrasound biomicroscopy has been used to evaluate the angle architecture
in some infants and children with congenital glaucoma.3,4 Such eyes often appear to have a thin iris and elongated ciliary processes, but
the angle is usually open and does not have any demonstrable
membrane. CORNEAL AND ANTERIOR SEGMENT DISORDERS In most patients, the anterior segment can be evaluated thoroughly by slit
lamp biomicroscopy unless the cornea is cloudy or opaque. In eyes
with a cloudy or opaque cornea, UBM can be used to evaluate the cornea
and to define the nature of underlying abnormalities in the angle, iris, ciliary
body, lens, and anterior vitreous.4 For example, in eyes with severe congenital malformations of the anterior
segment associated with a cloudy or opaque cornea (e.g., Peter's anomaly) (Fig. 14), UBM can be used to define the full extent of the abnormalities and thereby
aid the clinician in deciding whether or not to consider any surgical
intervention. UBM can also be used to study the extent of some
clinically evident corneal abnormalities, such as corneal edema, bullous
keratopathy, and band keratopathy (Fig. 15). In eyes with corneal edema (see Fig. 15A), UBM shows the epithelium to be thicker than normal and the stroma to
have increased reflectivity. In bullous keratopathy (see Fig. 15B), UBM shows epithelial blisters of the cornea. In band keratopathy (see Fig. 15C), UBM shows superficial calcific deposits that are strongly reflective
with shadowing of the underlying structures. In postinflammatory corneal
scarring (see Fig. 15D), UBM can show the nonuniform cross-sectional corneal thickness and the
presence or absence of a well-defined Descemet's membrane and endothelium
layer. 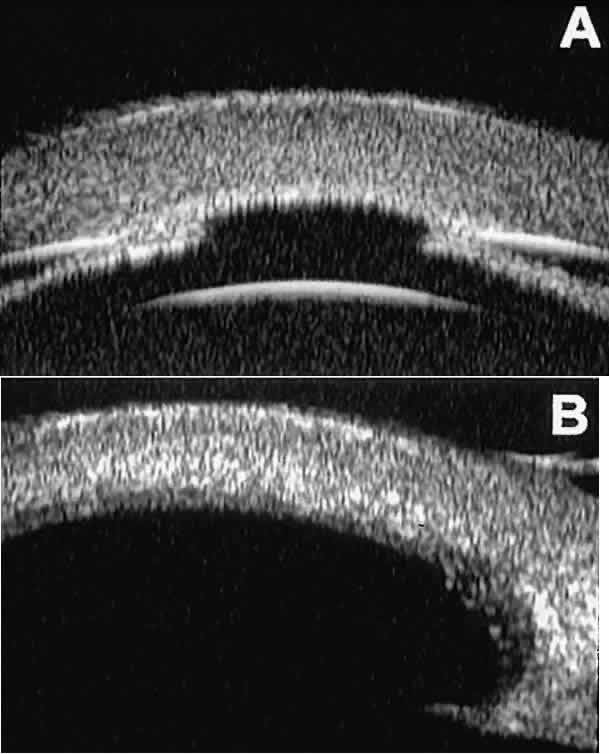 Fig. 14. UBM features of eyes with Peter's anomaly. A. Mild posterior central corneal excavation, absence of Descemet's
membrane and endothelium centrally, iridocorneal adhesions to margins
of corneal defect, and diffuse hyper-reflectivity of corneal stroma. B. Different patient showing detail of posterior central corneal excavation
and diffuse hyper-reflectivity of corneal stroma. Fig. 14. UBM features of eyes with Peter's anomaly. A. Mild posterior central corneal excavation, absence of Descemet's
membrane and endothelium centrally, iridocorneal adhesions to margins
of corneal defect, and diffuse hyper-reflectivity of corneal stroma. B. Different patient showing detail of posterior central corneal excavation
and diffuse hyper-reflectivity of corneal stroma.
|
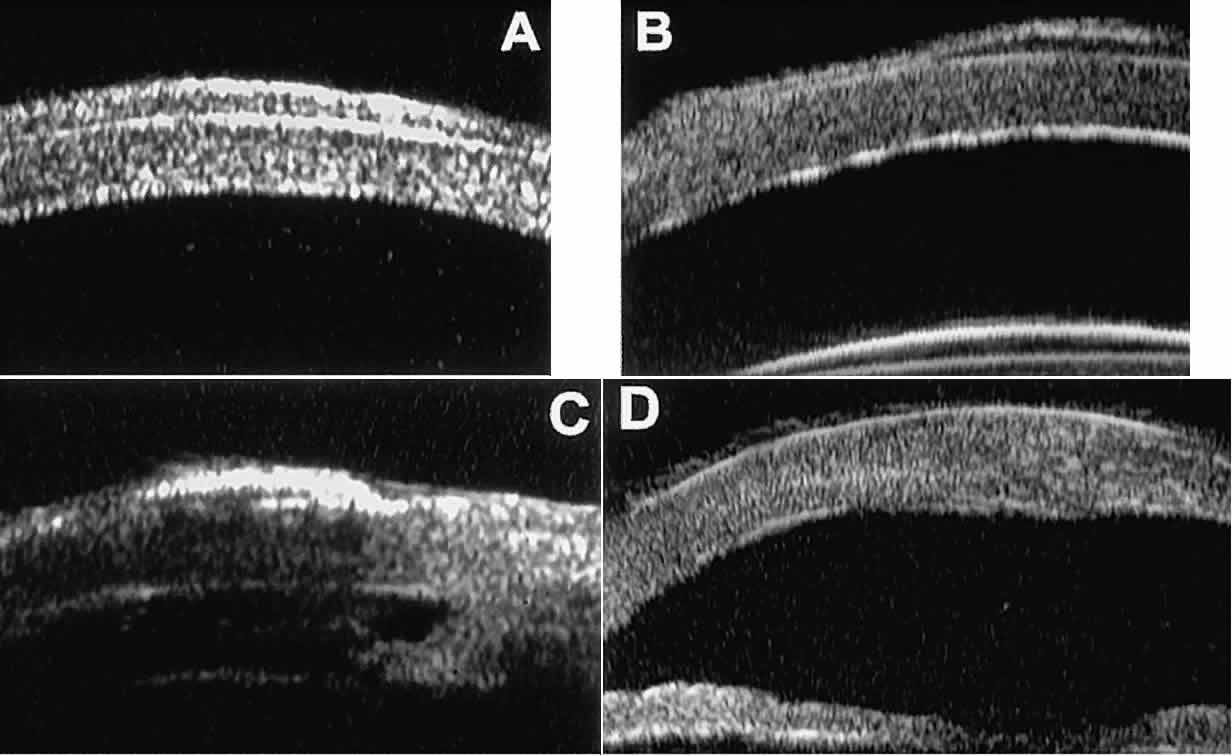 Fig. 15. UBM features of miscellaneous corneal disorders. A. Corneal edema appears as thickening of superficial layer of cornea; corneal
stroma is thinner than normal and abnormally bright. B. Bullous keratopathy appears as localized separation of corneal epithelium
from Bowman's membrane filled with clear serous fluid. C. Band keratopathy appears as dense, brightly reflective subepithelial plaque
in peripheral cornea. D. Postinflammatory corneal scarring after keratitis; note nonuniform corneal
thickness and abnormal reflectivity of corneal stroma. Fig. 15. UBM features of miscellaneous corneal disorders. A. Corneal edema appears as thickening of superficial layer of cornea; corneal
stroma is thinner than normal and abnormally bright. B. Bullous keratopathy appears as localized separation of corneal epithelium
from Bowman's membrane filled with clear serous fluid. C. Band keratopathy appears as dense, brightly reflective subepithelial plaque
in peripheral cornea. D. Postinflammatory corneal scarring after keratitis; note nonuniform corneal
thickness and abnormal reflectivity of corneal stroma.
|
Ultrasound biomicroscopy can be used after corneal surgery to evaluate
the anterior segment status. After corneal transplantation, UBM can be
used to evaluate the apposition of the donor button and host tissue and
to assess the presence or absence of vitreous or iris incarceration
in the incision. Ultrasound biomicroscopy has also been used to evaluate several anterior
scleral disorders,11 including nodular anterior scleritis and scleral hyaline plaques (Fig. 16). On UBM, nodular anterior scleritis (see Fig. 16A) appears as a localized thickening and altered reflectivity of the inflamed
sclera. In diffuse non-necrotizing anterior scleritis (see Fig. 16B), UBM shows generalized pronounced thickening of the sclera in the region
of involvement. In contrast, after a bout of necrotizing anterior
scleritis, UBM can show thinning of the damaged sclera (see Fig. 16C). In eyes with one or more scleral hyaline plaques (see Fig. 16D), UBM shows the lesion to be a highly sonoreflective plate located just
anterior to the insertion of the medial or lateral rectus muscle; the
lesion has well-defined margins and is so sonoreflective that it shadows
the underlying layers of the eye wall. 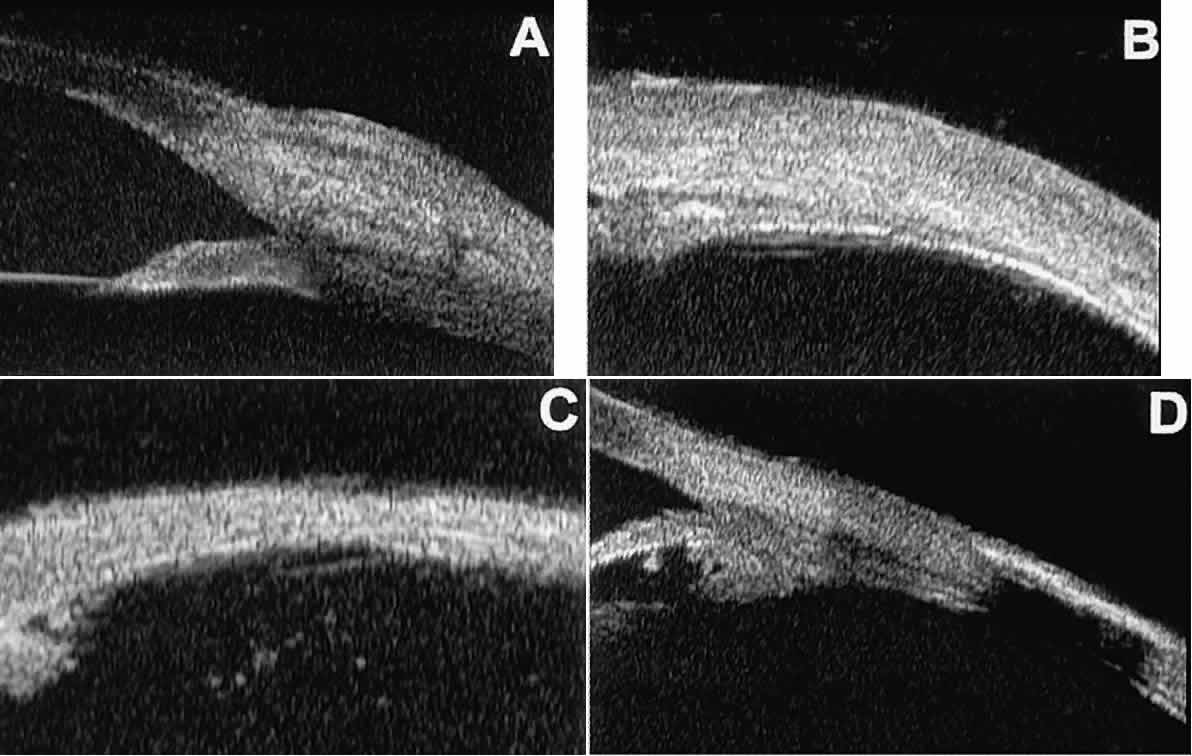 Fig. 16. UBM features of anterior scleral disorders. A. Nodular anterior scleritis appears as fusiform thickening of limbal sclera. Note
apparent lamellae of heterogeneous reflectivity within region
of thickening. B. Diffuse anterior scleritis appears as nonfocal scleral thickening in region
of inflammation. C. Scleral thinning subsequent to necrotizing anterior scleritis. Note underlying
vitreous cells. D. Scleral hyaline plaque appears as dense, hyper-reflective plate several
millimeters from horizontal limbus; dense lesion “shadows” deeper
tissues. Fig. 16. UBM features of anterior scleral disorders. A. Nodular anterior scleritis appears as fusiform thickening of limbal sclera. Note
apparent lamellae of heterogeneous reflectivity within region
of thickening. B. Diffuse anterior scleritis appears as nonfocal scleral thickening in region
of inflammation. C. Scleral thinning subsequent to necrotizing anterior scleritis. Note underlying
vitreous cells. D. Scleral hyaline plaque appears as dense, hyper-reflective plate several
millimeters from horizontal limbus; dense lesion “shadows” deeper
tissues.
|
CATARACT AND INTRAOCULAR LENS The role of UBM in the preoperative assessment of eyes with cataract is
as yet unknown. In certain eyes, however, UBM may reveal features or
abnormalities that could alter the ophthalmologist's surgical approach. Postoperatively, UBM can show the size and location of an intraocular
lens (IOL) and the positioning of the haptics. A posterior chamber
IOL appears on UBM as a highly reflective plate (corresponding to
the lens optic) in the retropupillary plane with reverberation artifacts
behind it (Fig. 17A). In contrast, an anterior chamber IOL appears on UBM as a sonoreflective
plate located anterior to the pupillary plane (see Fig. 17B). In most eyes with a posterior chamber IOL, UBM can show whether the
haptics are in the capsular bag (Fig. 18A), in the ciliary sulcus (see Fig. 18B), or in some other anatomic location12 (e.g., resting on the peripheral iris or secured with sutures to the sclera). The
haptics are easier to locate if they are made of polymethyl-methacrylate
than if they are made of proline because the former has a stronger
reflectance. 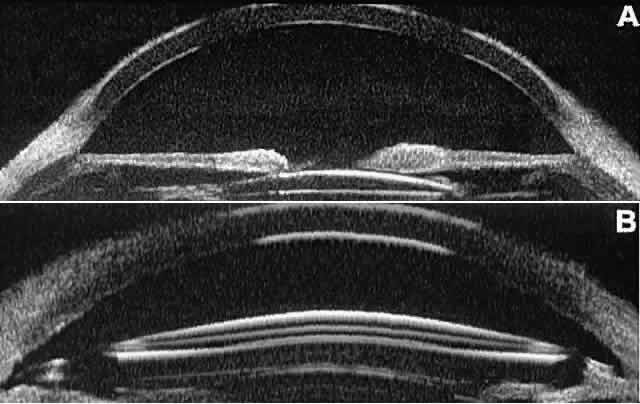 Fig. 17. Composite UBM images of intraocular lenses. A. Posterior chamber IOL. B. Anterior chamber IOL. Fig. 17. Composite UBM images of intraocular lenses. A. Posterior chamber IOL. B. Anterior chamber IOL.
|
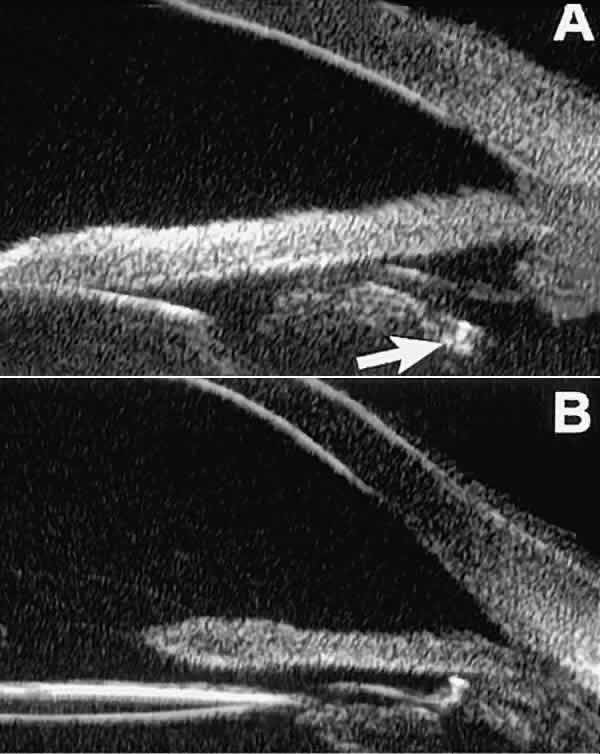 Fig. 18 . Localization of posterior chamber IOL haptics by UBM. A. Haptic in capsular bag (arrow). B. Haptic (bright object just behind peripheral iris) in iridociliary sulcus. Fig. 18 . Localization of posterior chamber IOL haptics by UBM. A. Haptic in capsular bag (arrow). B. Haptic (bright object just behind peripheral iris) in iridociliary sulcus.
|
Ultrasound biomicroscopy appears to be helpful postoperatively in determining
the extent of postoperative complications of cataract surgery such
as serous choroidal detachment (see Fig. 13A), iridocapsular adhesion (Fig. 19A), postoperative hyphema (see Fig. 19B), stripping of Descemet's membrane (see Fig. 19C), and wound gaping (see Fig. 19D). 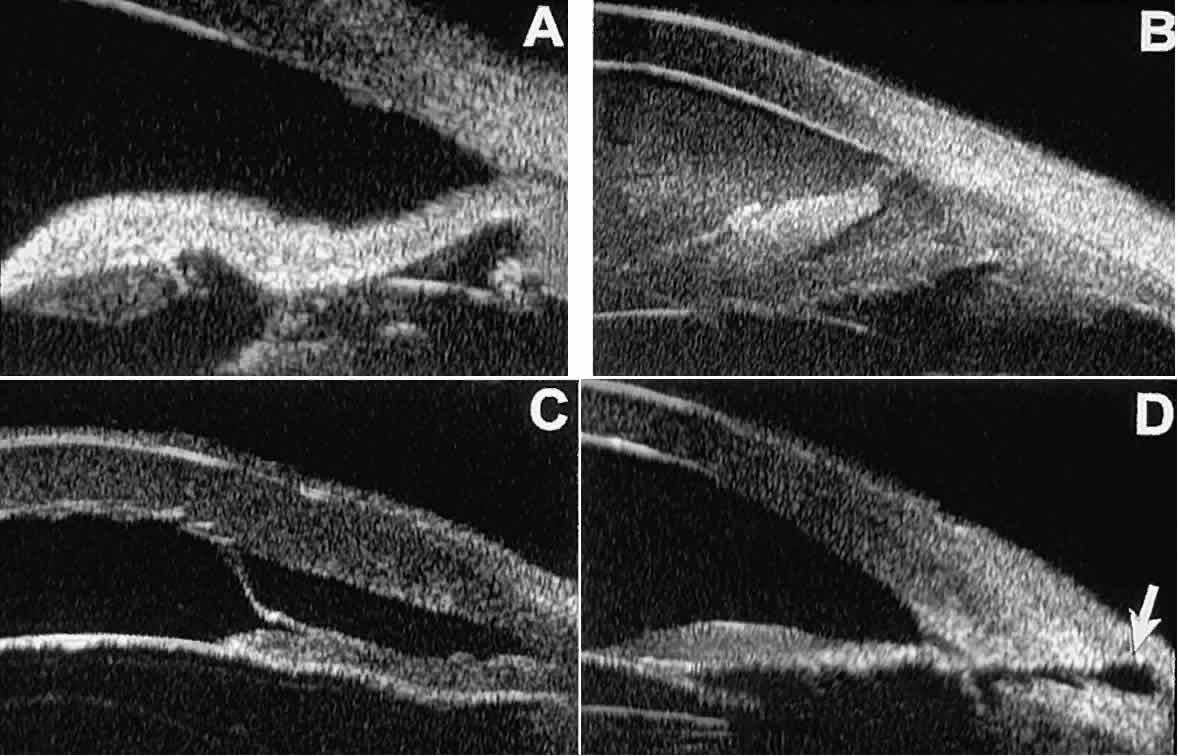 Fig. 19. Complications of cataract surgery revealed by UBM. A. Capsular adhesion to midzone of iris. B. Postoperative hyphema. Clot appears denser than aqueous with suspended
blood cells. C. Stripping of Descemet's membrane. D. Wound gape. Fig. 19. Complications of cataract surgery revealed by UBM. A. Capsular adhesion to midzone of iris. B. Postoperative hyphema. Clot appears denser than aqueous with suspended
blood cells. C. Stripping of Descemet's membrane. D. Wound gape.
|
UVEITIS In eyes with iritis or iridocyclitis, UBM can demonstrate many inflammatory
features in detail (Fig. 20).4 Inflammatory cells in the aqueous (see Fig. 20A) can be visualized by UBM as sonoreflective particles floating in the
sonolucent aqueous. Keratic precipitates appear on UBM (see Fig. 20B) as sonoreflective cellular clumps adherent to the endothelial surface
of the cornea. In hypopyon uveitis (see Fig. 20C), UBM shows the collection of white blood cells to be a dependent sonoreflective
mass in the anterior chamber with adherence to the peripheral
iris. Inflammatory nodules of the iris (see Fig. 20D) appear as relatively ill-defined, moderately sonoreflective lesions expanding
the normal iris stroma or lying superficially on it. 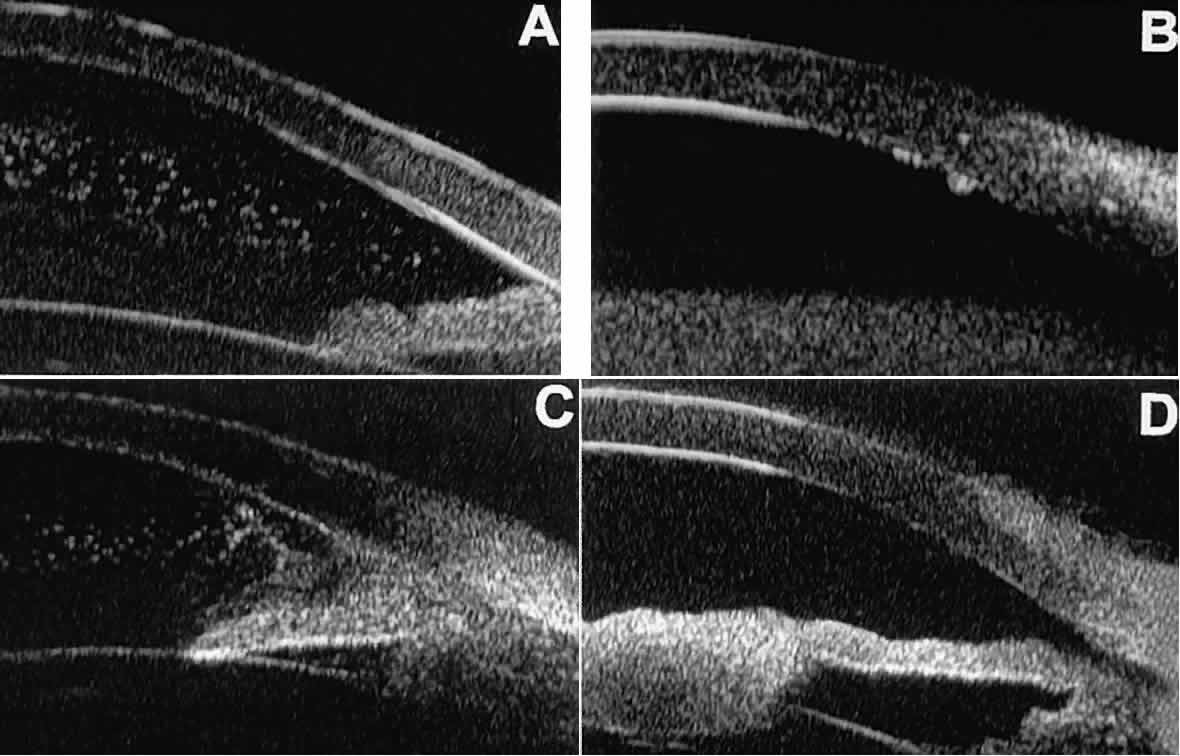 Fig. 20. UBM features of uveitis. A. Inflammatory cells suspended in anterior chamber aqueous. B. Keratic precipitates appear as small sonoreflective bumps on peripheral
corneal endothelium. C. Hypopyon. Mass of inflammatory cells fills inferior anterior chamber angle, and
dispersed cells are suspended in central anterior chamber aqueous. D. Inflammatory mass of pupillary zone of iris. Mass disappeared after corticosteroid
therapy. Fig. 20. UBM features of uveitis. A. Inflammatory cells suspended in anterior chamber aqueous. B. Keratic precipitates appear as small sonoreflective bumps on peripheral
corneal endothelium. C. Hypopyon. Mass of inflammatory cells fills inferior anterior chamber angle, and
dispersed cells are suspended in central anterior chamber aqueous. D. Inflammatory mass of pupillary zone of iris. Mass disappeared after corticosteroid
therapy.
|
TRAUMA After blunt ocular trauma, UBM can be used to evaluate iris-angle abnormalities
associated with and possibly obscured by hyphema, including angle
recession and cyclodialysis, and to illustrate the presence and extent
of blood clots.4 Angle recession is characterized on UBM (Fig. 21A) by posterior displacement of the point of attachment of the iris to the
sclera. In the acute stage, the post-traumatic recess is usually filled
with blood. Cyclodialysis (described and illustrated earlier) appears
on radial UBM slices through the limbal region (see Fig. 13B) as a fluid-filled cleft between the sclera and ciliary body.13 This abnormality is by definition associated with at least a localized
ciliochoroidal effusion. 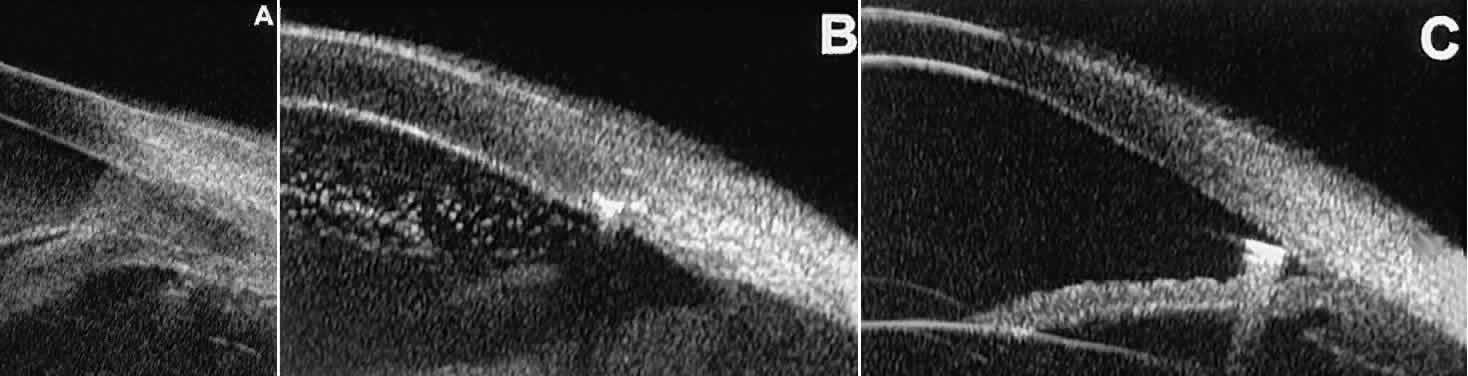 Fig. 21. UBM features of ocular trauma. A. Angle recession with traumatic hyphema after blunt injury. B. Intracorneal foreign body (rose thorn fragment). Note inflammatory cells
in adjacent aqueous. C. Intraocular foreign body (glass fragment in inferior angle). Fig. 21. UBM features of ocular trauma. A. Angle recession with traumatic hyphema after blunt injury. B. Intracorneal foreign body (rose thorn fragment). Note inflammatory cells
in adjacent aqueous. C. Intraocular foreign body (glass fragment in inferior angle).
|
After ocular perforations, lacerations, and intraocular surgery, UBM can
show abnormalities such as retained foreign bodies too small to be imaged
by other technologies.3,4 Foreign bodies appear on UBM (Fig. 22A and B) as highly reflective focal lesions that are frequently associated with
inflammatory features. 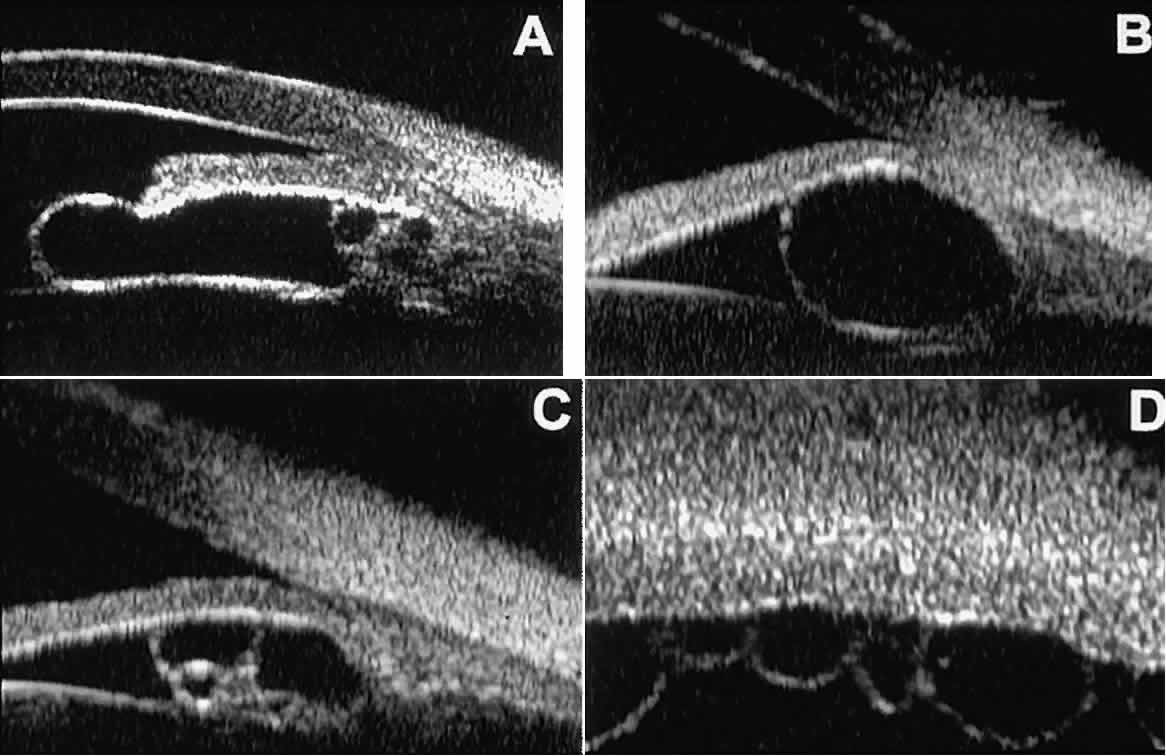 Fig. 22. UBM features of primary neuroepithelial cysts of iris and ciliary body. A. Primary neuroepithelial cyst of iris midzone. B. Primary neuroepithelial cyst of iridociliary sulcus. C. Multiple neuroepithelial cysts of peripheral iris and ciliary body. D. Neuroepithelial cysts of pars plana of ciliary body shown in circumferential
slice. Fig. 22. UBM features of primary neuroepithelial cysts of iris and ciliary body. A. Primary neuroepithelial cyst of iris midzone. B. Primary neuroepithelial cyst of iridociliary sulcus. C. Multiple neuroepithelial cysts of peripheral iris and ciliary body. D. Neuroepithelial cysts of pars plana of ciliary body shown in circumferential
slice.
|
OCULAR ONCOLOGY Cysts and solid tumors of the anterior segment can be imaged in great detail
with UBM.14,15 This technology can be used to determine the internal character of a lesion (solid
or cystic), to ascertain whether the lesion involves the
anterior ciliary body or is restricted to the iris, and to measure the
full extent of the lesion. Relatively small cysts and tumors of the anterior
segment can be imaged in their entirety by UBM. Larger lesions, however, may
not be fully imaged by UBM because of its limited depth
of penetration and relatively narrow field width. Cystic lesions of the iris and ciliary body can be of four types: primary
neuroepithelial cysts, stratified squamous epithelial cysts, neuroepithelial
cysts associated with solid tumors, and intratumoral cavities.15 Primary neuroepithelial cysts (see Fig. 22) are very distinct on UBM imaging. These lesions consist of a central
sonolucent cavity surrounded by a thin wall of highly reflective neuroepithelial
cells. They arise from the posterior surface of the iris (see Fig. 22A), in the iridociliary sulcus (see Fig. 22B and C), or from the inner aspect of the ciliary body (see Fig. 22D). They are often multifocal (see Fig. 22C and D) and bilateral.15 The largest lesions of this type typically occur in or near the horizontal
meridians. Stratified squamous epithelial cysts (Fig. 23) are almost exclusively unilateral and unifocal,15 have substantially thicker walls than do primary neuroepithelial cysts, and
usually contain prominent intracavitary particles (desquamated epithelial
cells). Almost all such cysts involve the peripheral iris and
angle region. Such cysts are usually secondary to prior ocular surgery
or laceration in which conjunctival epithelial cells were implanted
into the iris stroma. 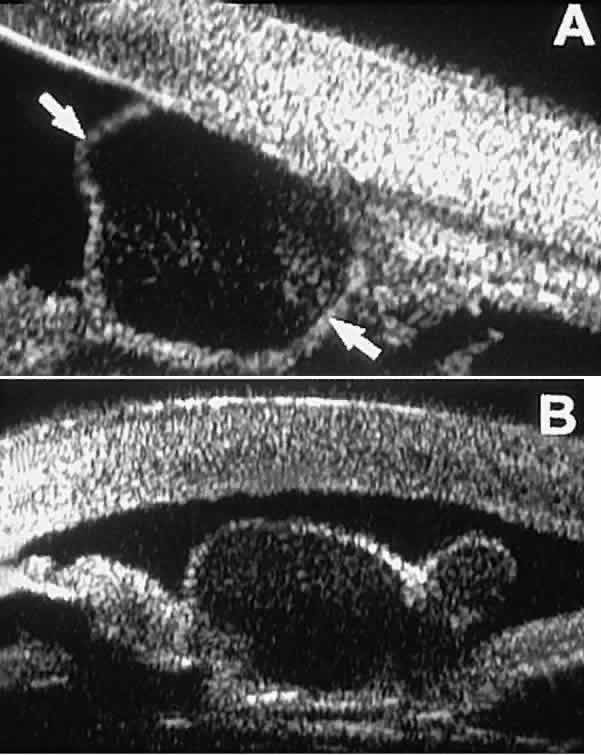 Fig. 23. UBM features of stratified squamous epithelial cysts of iris. A. Thick-walled implantation cyst of stratified squamous epithelium replacing
normal iris. Note intracavitary particles. B. Bilobed stratified squamous epithelial inclusion cyst of iris with prominent
intracavitary particles. Fig. 23. UBM features of stratified squamous epithelial cysts of iris. A. Thick-walled implantation cyst of stratified squamous epithelium replacing
normal iris. Note intracavitary particles. B. Bilobed stratified squamous epithelial inclusion cyst of iris with prominent
intracavitary particles.
|
Secondary neuroepithelial cysts occur rather frequently in association
with solid tumors of the iris or ciliary body.15 On UBM (Fig. 24), such cysts appear quite similar to the primary neuroepithelial cysts
described above; however, they are associated with a solid mass arising
within the iris or ciliary body. 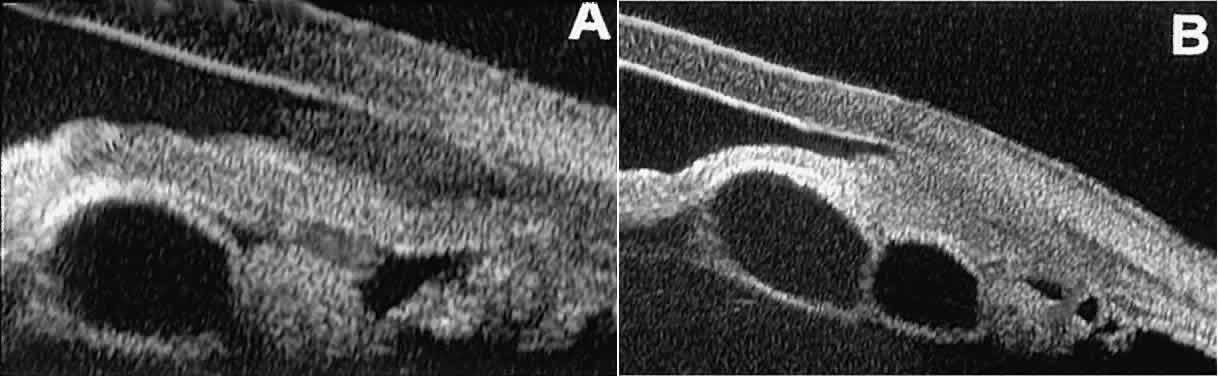 Fig. 24. UBM appearance of neuroepithelial cysts associated with solid tumors of
the iris and ciliary body. A. Single neuroepithelial cyst associated with iris melanoma. B. Multiple neuroepithelial cysts associated with iridociliary melanoma. Fig. 24. UBM appearance of neuroepithelial cysts associated with solid tumors of
the iris and ciliary body. A. Single neuroepithelial cyst associated with iris melanoma. B. Multiple neuroepithelial cysts associated with iridociliary melanoma.
|
Intratumoral cavitation is a relatively uncommon cystic feature of some
solid tumors. An intratumoral cavity appears on UBM as a well-defined
sonolucent space within the stroma of the solid tumor (Fig. 25).15 The lack of pulsation during the UBM examination of such lesions and the
diameter of the cavity enable the clinician to differentiate the cavitation
from a large blood vessel.  Fig. 25. Cavitation within iridociliary melanoma revealed by UBM. The cavity is
entirely sonolucent, and the tumor tissue adjacent to the cavity appears
similar in reflectivity to that in other areas of the tumor. Fig. 25. Cavitation within iridociliary melanoma revealed by UBM. The cavity is
entirely sonolucent, and the tumor tissue adjacent to the cavity appears
similar in reflectivity to that in other areas of the tumor.
|
Solid iridociliary tumors present variable internal reflectivity depending
on tumor type.14 Most solid lesions that occur on the iris are nevi. Benign nevi of the
iris and ciliary body usually appear on UBM as relatively small hyporeflective
lesions replacing a part or all of the underlying uveal stroma
locally (Fig. 26). Such lesions usually do not destroy the underlying neuroepithelium of
the iris or ciliary body, extend intrasclerally, or have prominent intralesional
blood vessels. 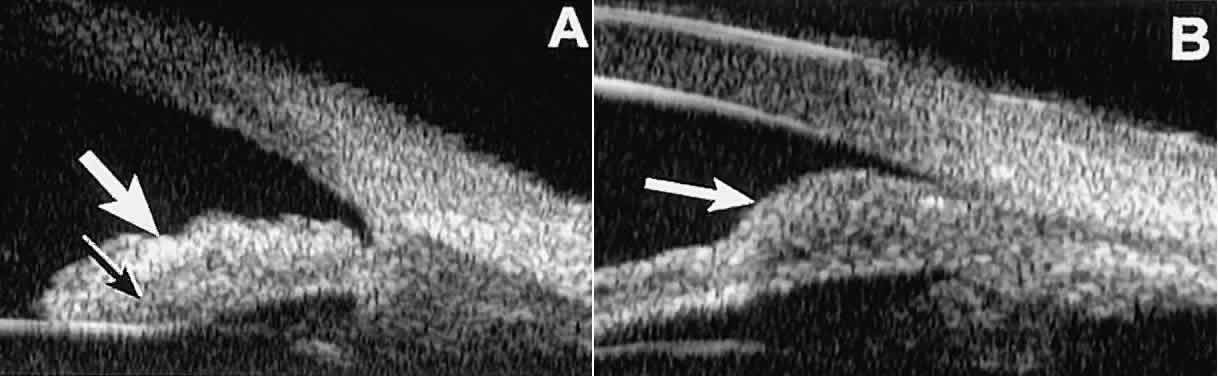 Fig. 26. UBM features of iris nevi. A. Superficial nevus appears as hyper-reflective layer of iris (white arrow). Normal iris stroma (dark arrow) is more sonolucent. B. Fusiform nevus of peripheral iris occupying full thickness of iris stroma (arrow). Note intact iris pigment epithelium underlying lesion. Fig. 26. UBM features of iris nevi. A. Superficial nevus appears as hyper-reflective layer of iris (white arrow). Normal iris stroma (dark arrow) is more sonolucent. B. Fusiform nevus of peripheral iris occupying full thickness of iris stroma (arrow). Note intact iris pigment epithelium underlying lesion.
|
In contrast, malignant melanomas of the iris and ciliary body are much
less common. On UBM, such tumors are usually larger than benign nevi, and
they are more likely to have caused focal or extensive disruption
of the adjacent neuroepithelial layers, to have invaded the sclera, and
to be associated with prominent intralesional blood vessels (Fig. 27). Some malignant melanomas of the ciliary body and most ciliochoroidal
melanomas are too large in basal diameter to be fully revealed in a single
UBM image, and many of these lesions are also too thick to be measured
by this technology. In the case of a melanocytic tumor of the iris
or ciliary body that is not clearly either a benign nevus or a malignant
melanoma, serial UBM evaluations may prove useful for assessing
the tumor's growth and other changes that might warrant either biopsy
or complete excision of the mass. 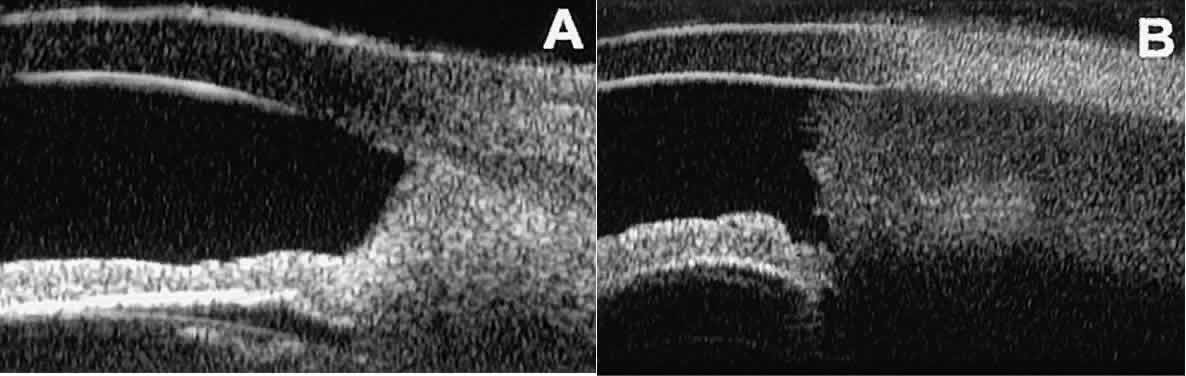 Fig. 27. UBM features of malignant melanoma of iris. (A) Iridociliary melanoma replacing peripheral iris and ciliary body and filling
anterior chamber angle. Mass is slightly sonolucent compared with
normal iris stroma. (B) Larger iridociliary melanoma. Iris appears to arise from side of mass. Fig. 27. UBM features of malignant melanoma of iris. (A) Iridociliary melanoma replacing peripheral iris and ciliary body and filling
anterior chamber angle. Mass is slightly sonolucent compared with
normal iris stroma. (B) Larger iridociliary melanoma. Iris appears to arise from side of mass.
|
UBM has been used to evaluate various solid epibulbar masses, including
limbal dermoids, squamous cell carcinomas of the conjunctiva, conjunctival
cysts, and episcleral extensions of ciliary bodyneoplasms (Fig. 28).3 A limbal dermoid appears on UBM as an intensely sonoreflective mass involving
and replacing the corneal and scleral stroma at the limbus (see Fig. 28A). The UBM images can reveal whether the lesion involves only partial thickness
or full thickness of the stroma and can thereby aid in surgical
planning. An acquired epibulbar neoplasm, such as squamous cell carcinoma
of the conjunctiva and its variants, typically appears on UBM as
an irregular, abnormally sonoreflective epibulbar mass adjacent to normal
conjunctiva (see Fig. 28B and C). UBM allows measurement of the lesion's thickness and determination
of the presence or absence or intraocular invasion (see Fig. 28D). Extrascleral extension of a ciliary body melanoma can simulate a conjunctival
melanoma in some cases. UBM of such eyes confirms the presence, character, and
extent of the underlying ciliary body tumor and often
reveals the route of access of the tumor to the surface by way of a
scleral emissary canal (see Fig. 28D).14 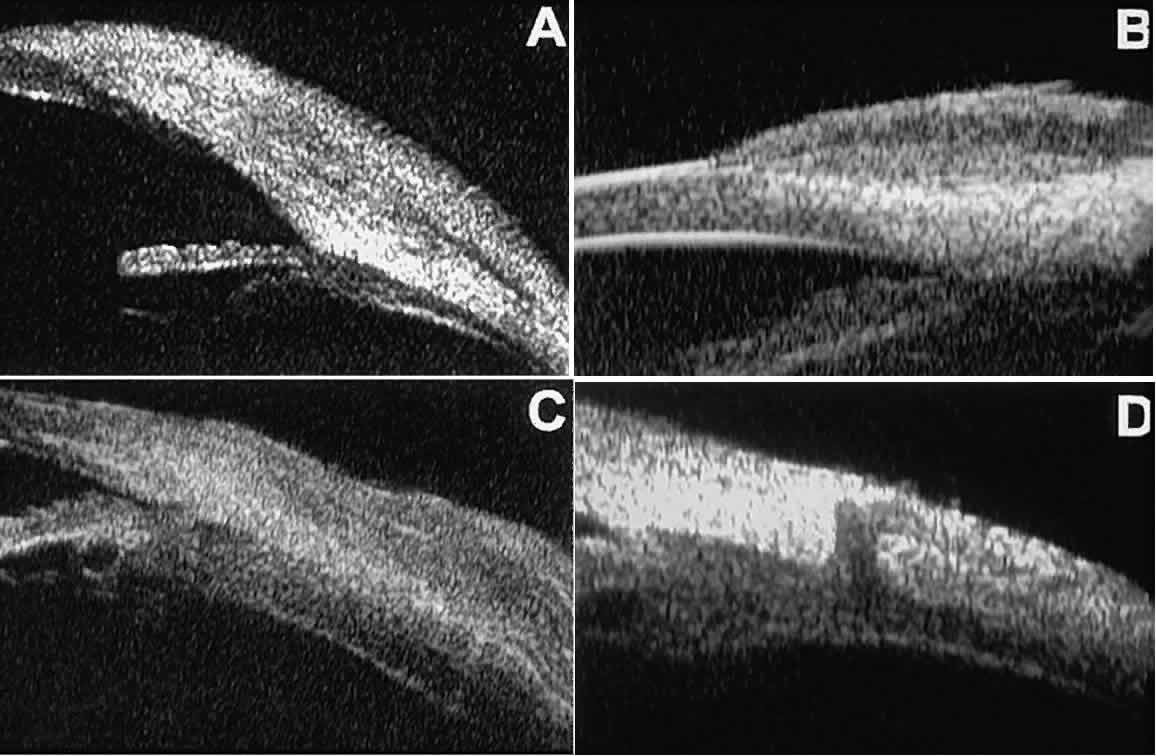 Fig. 28. UBM features of epibulbar mass lesions. (A) Composite UBM image of limbal dermoid. Lesion is sonoreflective and appears
to replace full-thickness limbal cornea and sclera. (B) Squamous cell carcinoma of conjunctiva without intraocular invasion. Mass
appears as fusiform thickening of limbal conjunctiva. (C) Squamous cell carcinoma of conjunctiva with scleral invasion. Invaded
sclera appears abnormally sonolucent and nonuniform in thickness. (D) Extrascleral extension of ciliary body melanoma by way of scleral vascular
or neural foramen. Fig. 28. UBM features of epibulbar mass lesions. (A) Composite UBM image of limbal dermoid. Lesion is sonoreflective and appears
to replace full-thickness limbal cornea and sclera. (B) Squamous cell carcinoma of conjunctiva without intraocular invasion. Mass
appears as fusiform thickening of limbal conjunctiva. (C) Squamous cell carcinoma of conjunctiva with scleral invasion. Invaded
sclera appears abnormally sonolucent and nonuniform in thickness. (D) Extrascleral extension of ciliary body melanoma by way of scleral vascular
or neural foramen.
|
|