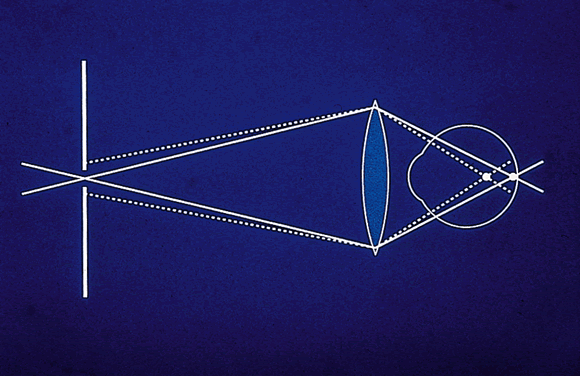
cSLOs represent an adaptation of confocal microscopy, an established imaging technology. The basic principle of confocal imaging is to illuminate through a single pinhole and image this point source on the object of interest. The light returns through the imaging optics and passes through a pinhole in a plane conjugate to the point of interest. Only light that returns from the point of interest can pass through the pinhole and be detected. Light from any point not in that focal plane will be greatly attenuated (Fig. 1). To image a plane rather than a point, an array of points in that plane must be scanned point by point. In a cSLO system, the illuminating light spot can be rapidly moved (scanned) across, resulting in a focal plane of data points or an optical section. This horizontal (x) and vertical (y) scanning is conducted using oscillating mirrors. Depth (z) is controlled (i.e., the focal plane is changed) by moving the objective lens. A transfer function is used to establish the location along the z-axis of maximum reflectivity. This is assumed to be the height at that coordinate and is used to generate the topography image.1
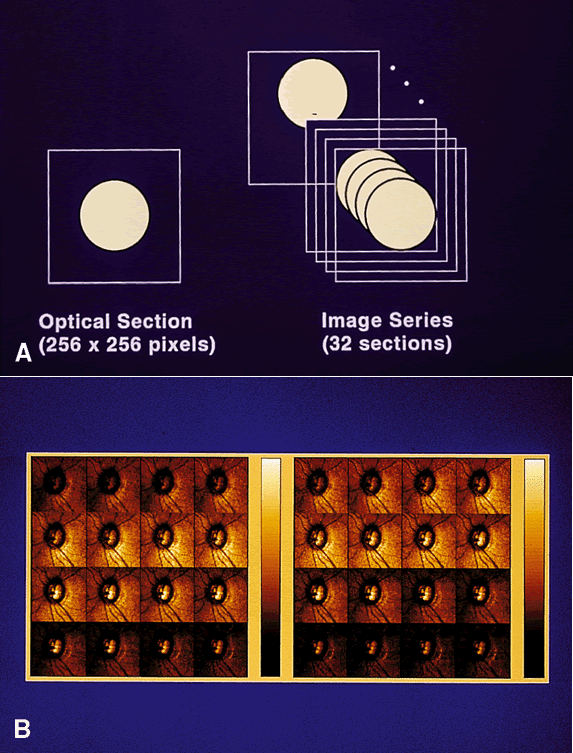
The cSLO generates a robust data set. Using a 256 × 256 matrix and 32 optical sections, the data set is approximately 65,000 height values from more than 2 million data points. When one considers that automated threshold perimetry generates only 72 discrete values, it is clear that powerful analysis strategies are needed to interpret the data. There are many approaches to data analysis. For example, data can be reviewed by pixel, region, or a derived parameter such as neuroretinal rim area. Most current strategies use only a subset of the 65,000 values.
A compact laser diode is used as the illumination source. Images can be obtained with light intensities as little as one thousandth of typical fundus flash illumination. The optical resolution is limited by the optics of the eye to about 10 μm transversely and 300 μm longitudinally.2 However, longitudinal measurements can be determined more accurately than this. If the serial optical sections are spaced closely, the height measurement can be determined from the plane of maximum reflectivity.
Clinically practical laser scanning optic nerve tomography first became possible in 1986 with the laser tomographic scanner (Heidelberg Instruments, Heidelberg, Germany). This instrument was used principally as a research tool and served as the prototype for the current generation of instruments. There are currently two commercially available cSLO systems—the Heidelberg retina tomograph (HRT; Heidelberg Engineering) and the topographic scanning system (Laser Diagnostic Technologies, Inc., San Diego, CA). Although both systems are based on the principles described above, there are hardware and software differences between them such as laser wavelength and definition of reference surface that makes direct comparisons difficult. No studies have directly compared the two instruments. These systems have been described in detail elsewhere.1–3
For typical optic nerve topographic measurement, the image series height range is defined by the operator to begin above the retinal plane and to terminate below the deepest point of the optic cup. Acquisition of a series of 32 images takes about 1 to 1.6 seconds, depending on the system used (Fig. 2). Examination can be performed undilated or dilated. Current practice is to obtain at least three images per session. Dilation is not needed if the pupil is at least 2.5 mm and the media are clear.
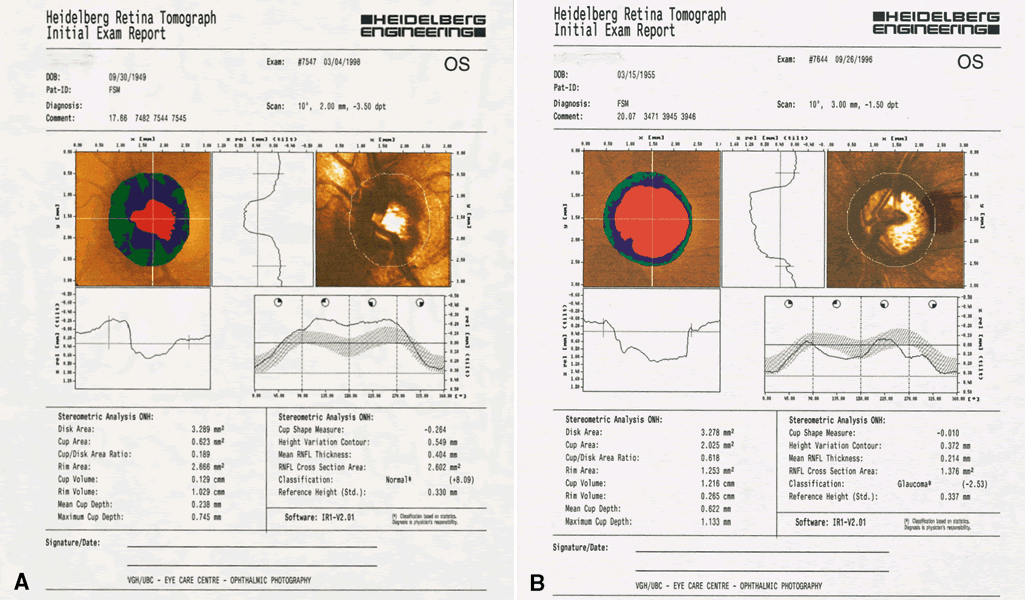
The 32 optical sections are processed to create a map with a resolution of 256 × 256 pixels. These 65,536 pixel values represent the height in the image relative to a reference plane (Fig. 3). This matrix of values can be analyzed using software included with the commercial systems, or the raw data can be exported and subjected to research strategies.
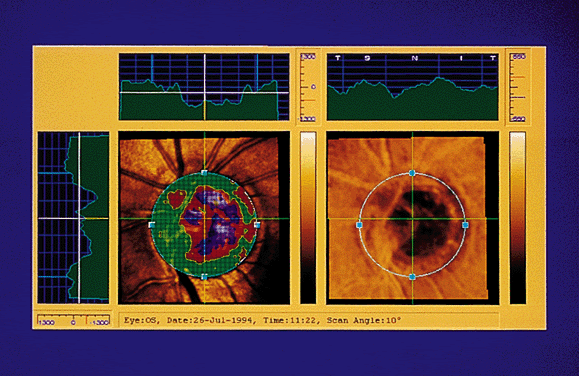
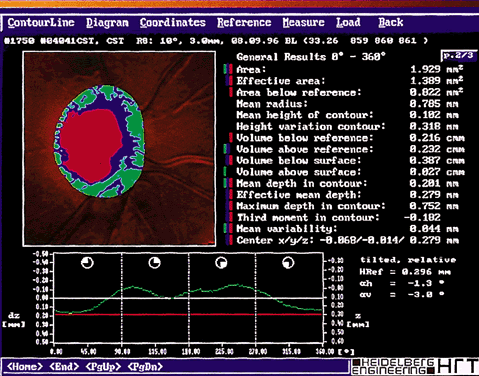
Once the images have been captured and before the system can compute topographic parameters, the operator must mark the area of interest, usually the optic nerve boundaries (Fig. 4). The computer establishes a retinal reference plane, then compares the depth measurements within the area of interest with respect to the reference plane (Fig. 5). The topographic data can be stored along with the area of interest data for comparison with subsequent images. Raw data can also be stored.
|
There are two principal potential applications for ONH analysis in the diagnosis and treatment of glaucoma: (1) distinguishing between normal and pathologic ONHs and (2) identifying progressive change with sequential images of the same ONH. Essential to both these applications is an accurate and reproducible technology.