TRUE EXFOLIATION
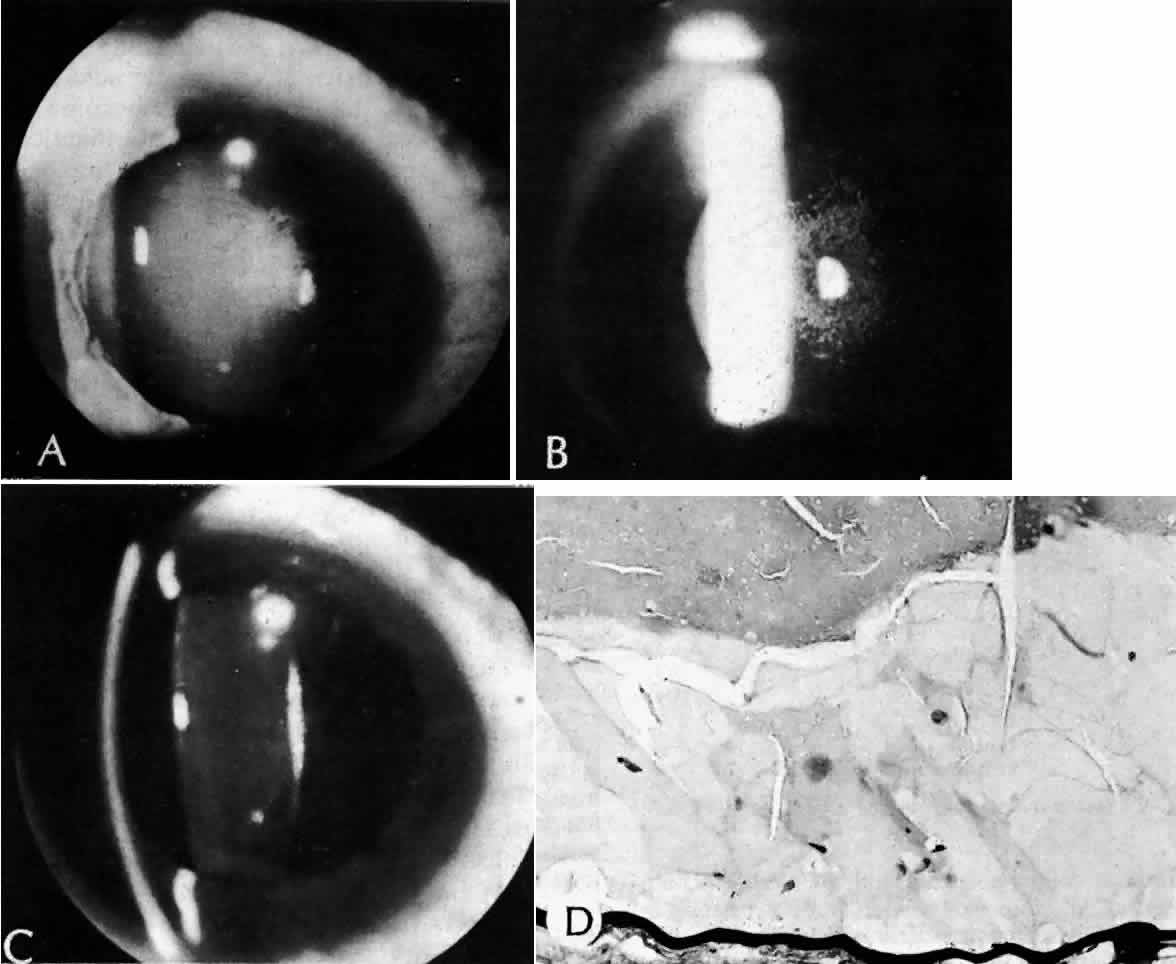
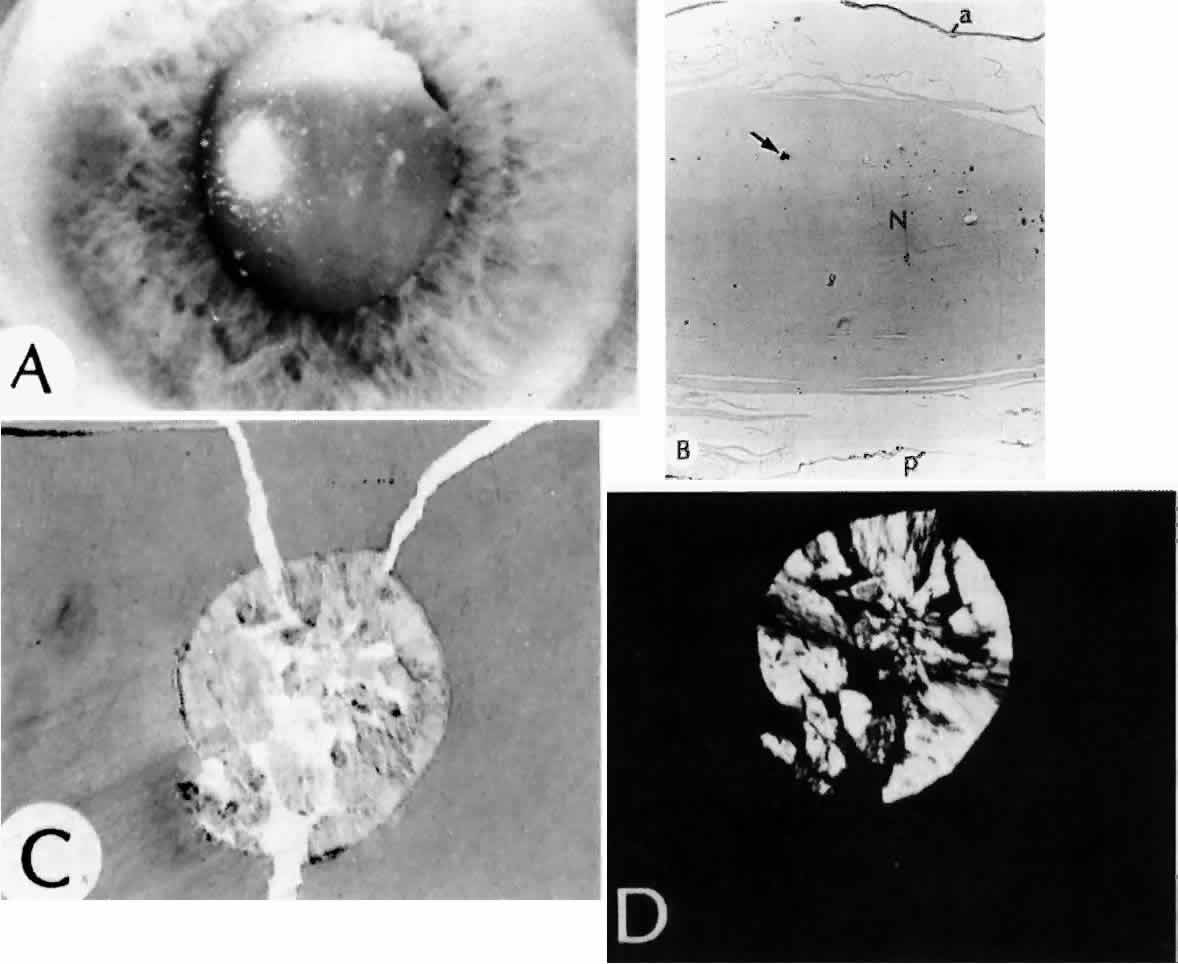
True exfoliation of the lens capsule is a condition in which the anterior aspect of the capsule splits. Clinical findings are a thin, clear, colorless membrane floating in the pupillary axis. It was first described in glass blowers and was believed to be a consequence of the considerable infrared radiation to which they were exposed. It has also been described in blacksmiths and steelworkers as well as in other persons who have had similar infrared exposure.27
It is possible, however, for patients to develop true exfoliation without having an apparent history of infrared radiation exposure. True exfoliation secondary to severe iridocyclitis or occurring as a sequela of trauma has been suggested.28 In some cases, the cause is obscure, but it may be a change associated with aging. In the largest reported series of 11 eyes in seven patients, the youngest patient was 79. All were hyperopic.27
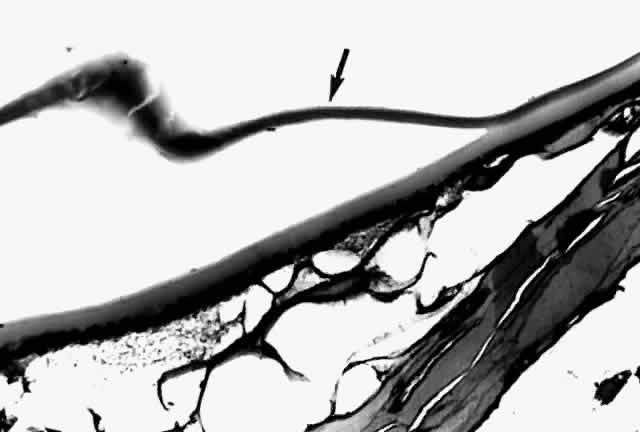
Histologic examination shows an unequal splitting of the anterior capsule (Fig. 1). Electron microscopic examination shows that the lens capsule is laminated and the anterior layer or layers are split free.29 The free portion is quite thin, and it can be rolled or folded over.27
PSEUDOEXFOLIATION SYNDROME
Pseudoexfoliation of the lens is much more common than true exfoliation. The term is actually a misnomer, as it has become clear that the origin of the pseudoexfoliation material is not the lens capsule. It is more prevalent in Scandinavian countries and is associated with secondary open-angle glaucoma.30
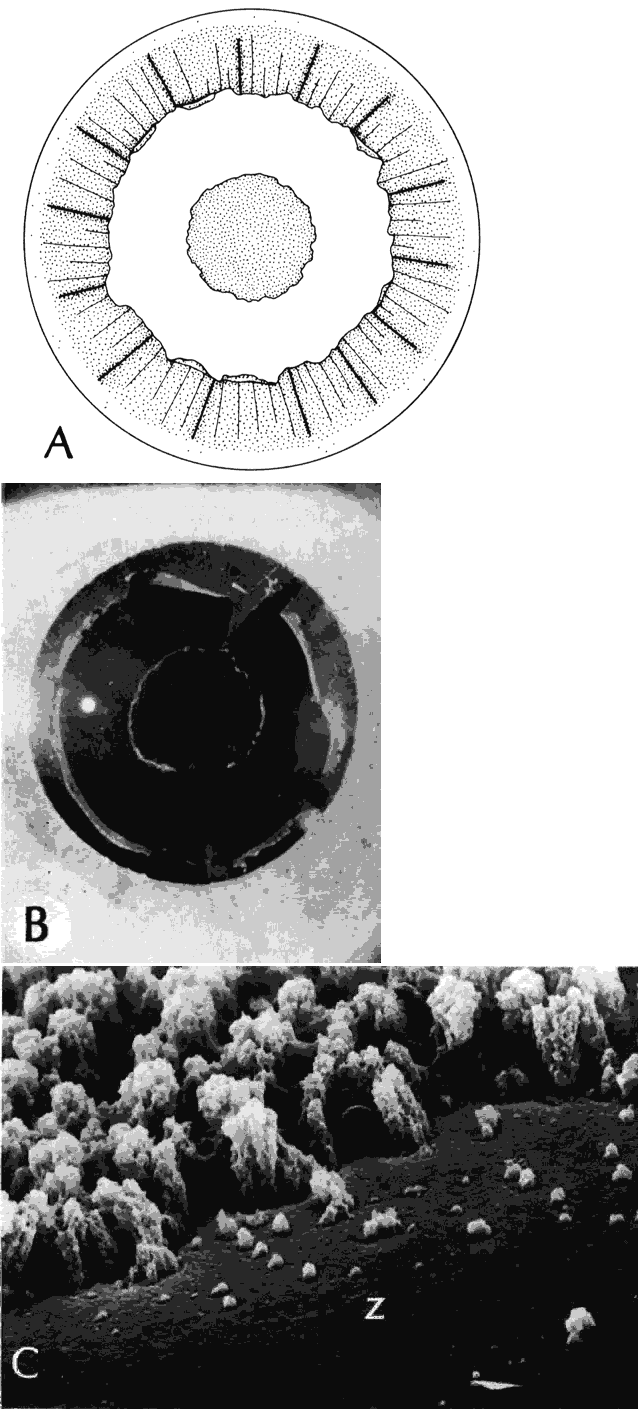
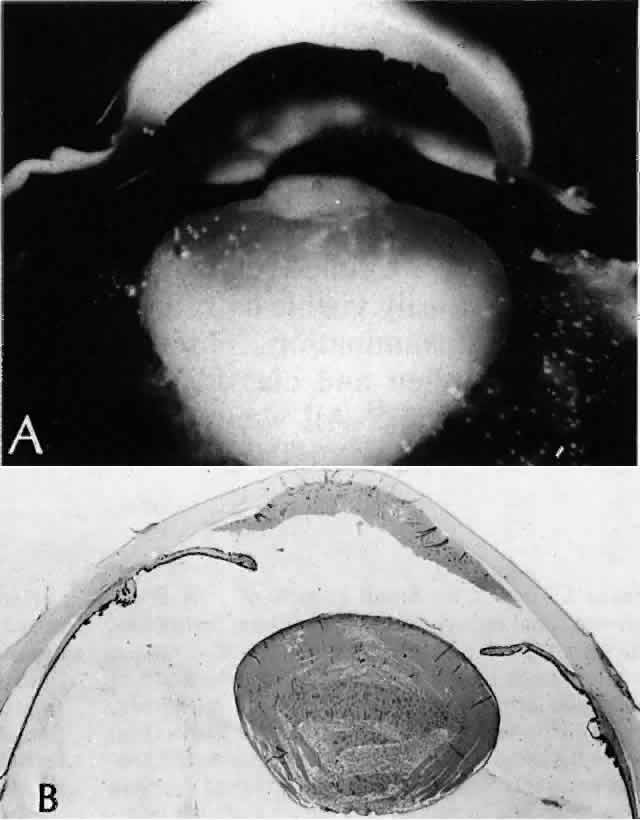
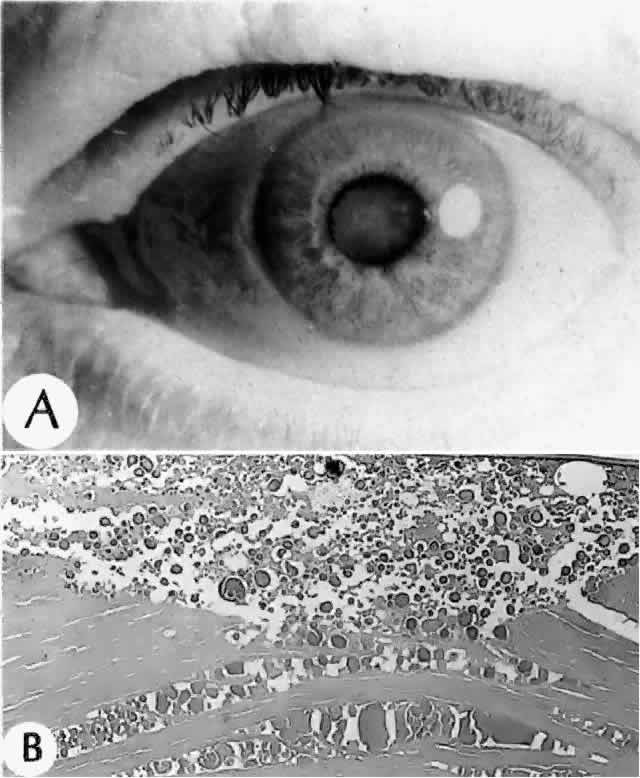
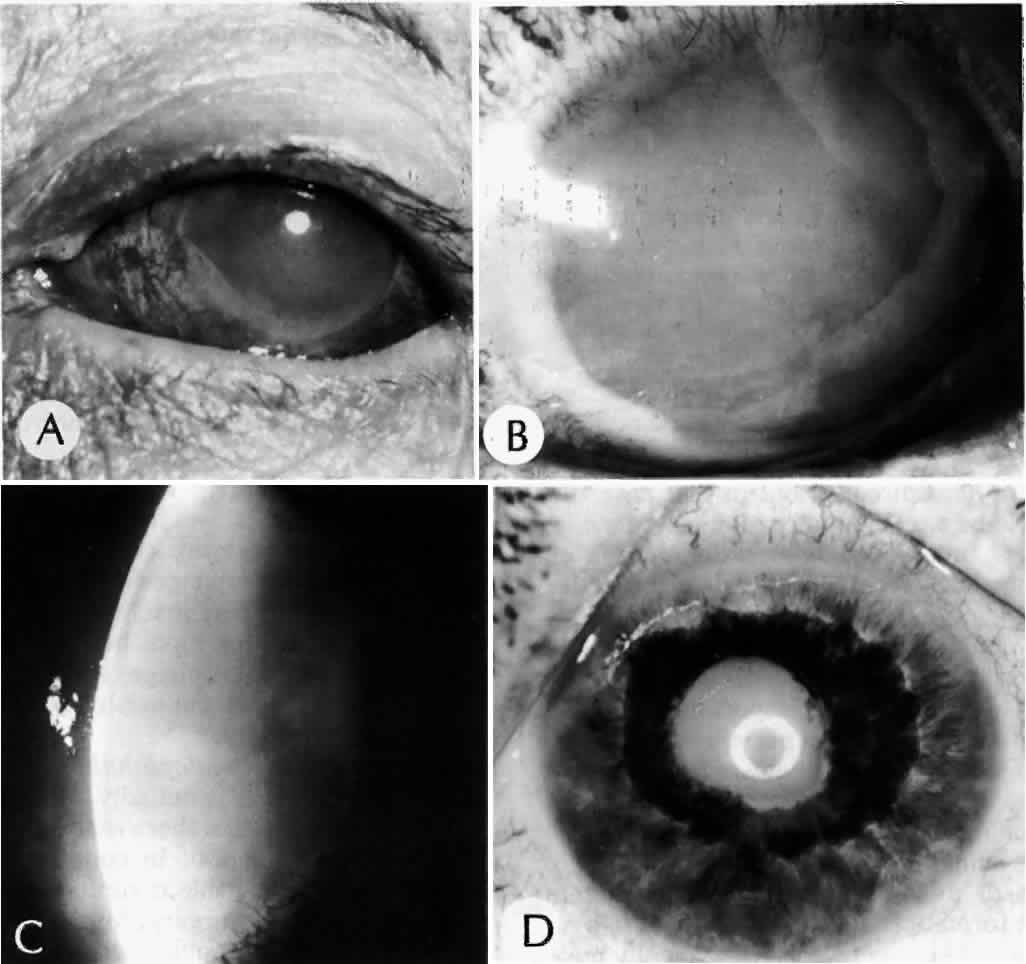
Clinically, in its classic manifestation, pseudoexfoliation syndrome is manifest as a whitish, fluffy deposit on the lens surface and at the pupillary margin. Typically there is a circular clear zone in the midperiphery of the lens, where the iris comes in contact with it (Fig. 2). This syndrome is important to recognize because a type of secondary open-angle glaucoma that can be difficult to control is associated with pseudoexfoliation in more than 20% of eyes.31 Other associated changes include poor dilatation of the iris, melanin dispersion from degeneration of the iris pigment epithelium, and iris stromal atrophy, which are visible clinically as transillumination defects.32
Because of its appearance, it was natural to assume that the exfoliation material was derived from the lens capsule. In some eyes, however, it was observed to persist or to develop long after intracapsular lens extraction. The stimulus for its deposition is likewise unknown. Subsequently, the material has been found in the conjunctiva,33 iris,32 and visceral organs.31 Thus, pseudoexfoliation syndrome appears to be a systemic disease, although there are no clinical symptoms apart from those in the eye. The syndrome has been described as typically unilateral, but it now appears that it is more frequently bilateral but asymmetric. Sometimes, too, eyes with uncontrolled open-angle glaucoma and other signs of exfoliation, such as poorly reactive pupils, but with no evidence of intraocular exfoliation material, can be shown to have the syndrome by conjunctival biopsy.33
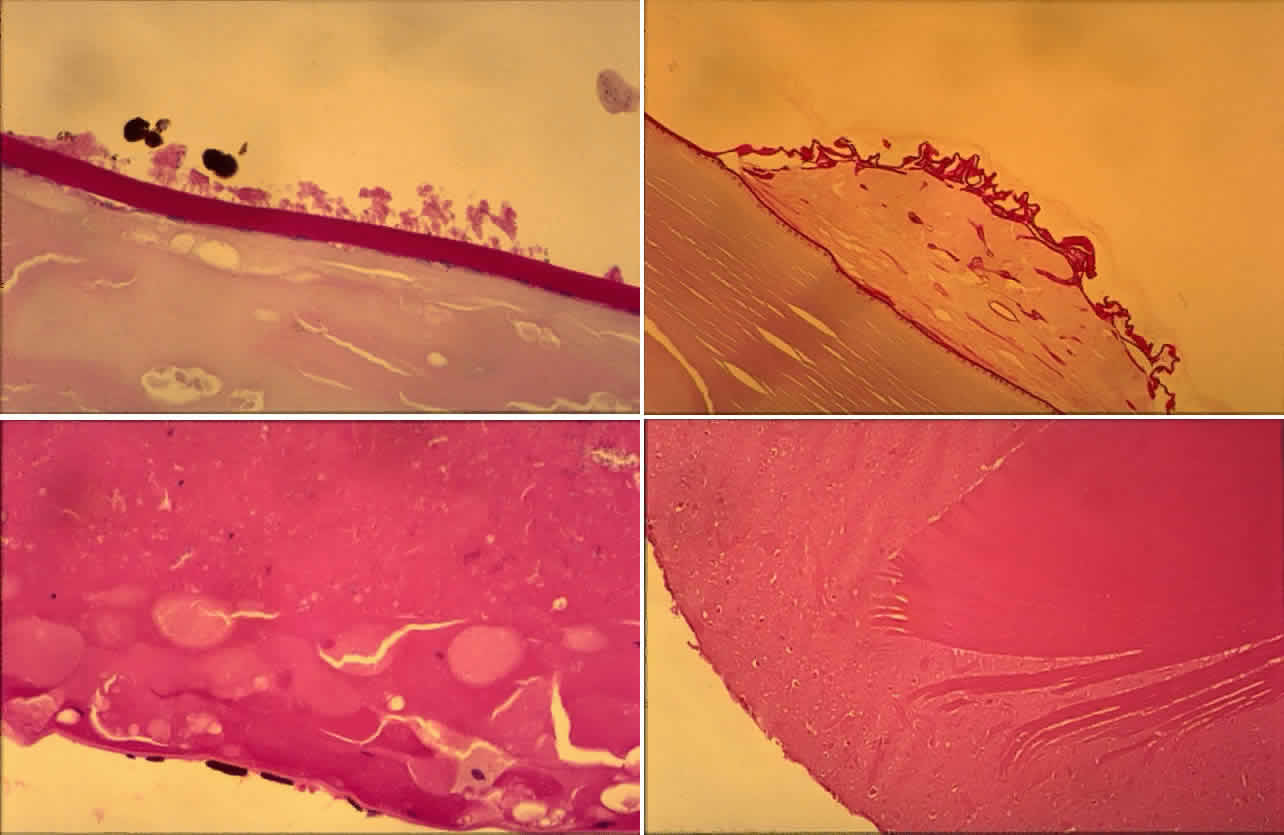
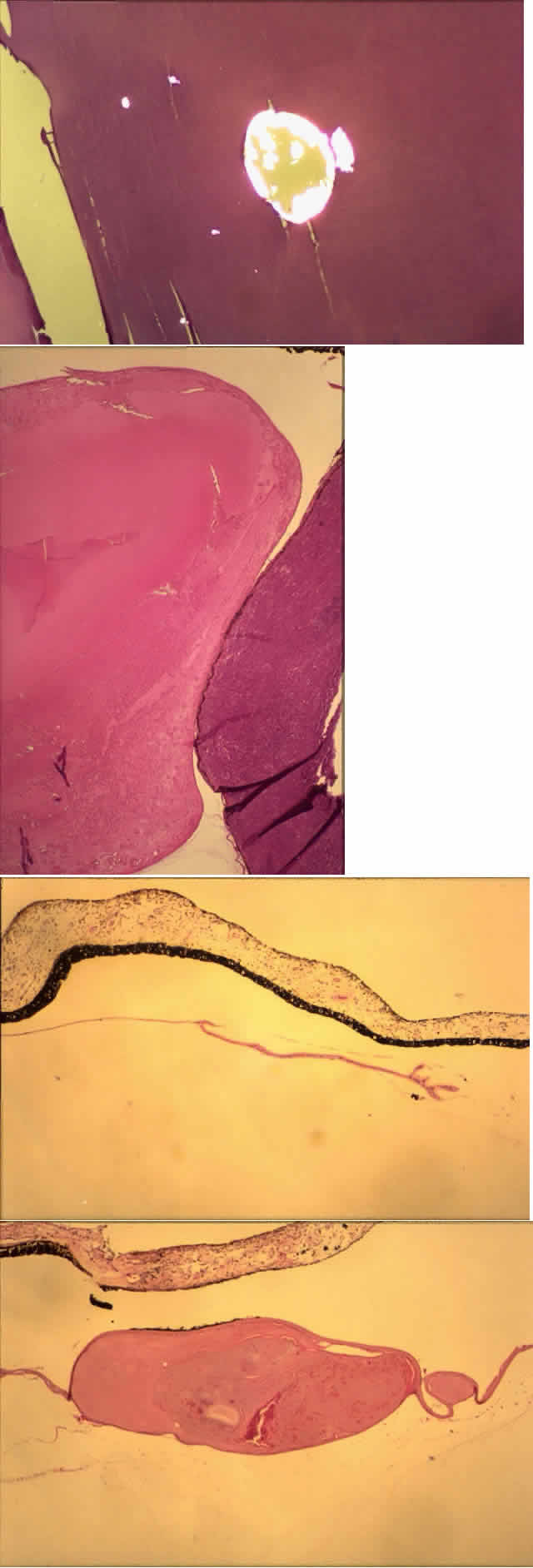
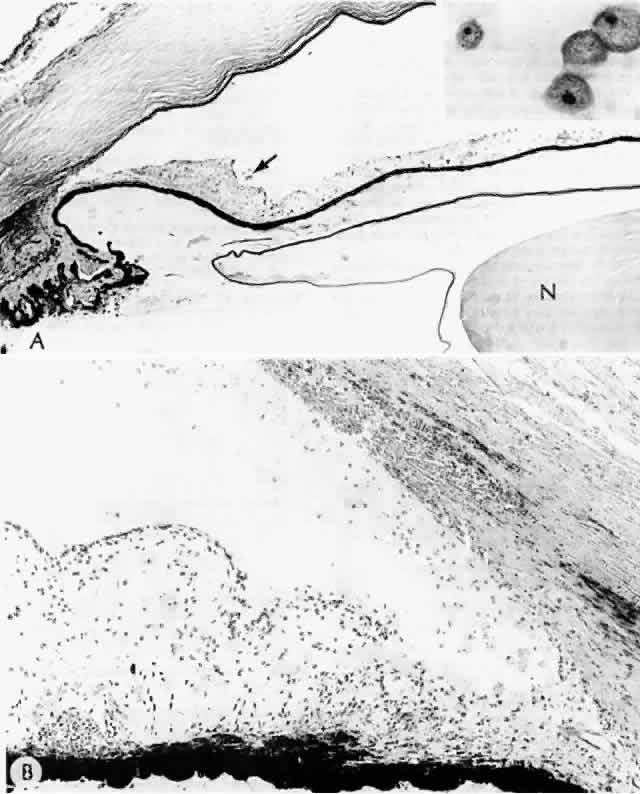
Histologic examination shows exfoliation deposits appearing as short strands of eosinophilic material, which are found on the surface of the lens and zonules and within and adjacent to the ciliary body and iris (Color Plate 1A). On electron microscopic examination, these strands are found to be thick, straight, and densely osmiophilic, with associated thin microfibrils.33
The iris pigment epithelium takes on a characteristic jagged, sawtooth appearance as melanin granules are lost from the cells. Degenerative changes with accumulations of the pseudoexfoliation fibers can be seen in both the sphincter and dilator muscles, probably accounting for the poor reactivity of the pupil.32
Recently, the pseudoexfoliation syndrome has been identified as a risk factor for lens capsule dislocation and vitreous loss during cataract surgery. On clinical examination, the zonules appear weakened by the exfoliation material. Electron microscopic examination reveals accumulated material along the length of the zonule and also at the origin and insertion of the zonules. Aggregates of exfoliation material interfere with the normal attachment of the zonules and interrupt the length of the zonule. Lysosomal enzymes are present within pseudoexfoliation fiber aggregates, presumably facilitating zonular dissolution.34
The exact nature of the pseudoexfoliation material has not been discerned. Probably it is an abnormal metabolite or component of basement membrane material. Many associated substances have been identified by immunostaining, including proteins of elastic tissue (e.g., elastin, fibrillin, amyloid P) and basement membrane-related components (e.g., chondroitin sulfate).31 Antibody testing has identified specific carbohydrate moieties in pseudoexfoliation material that are also present in various types of adhesion molecules.35 The cause and mechanism of this disease, however, remain elusive.