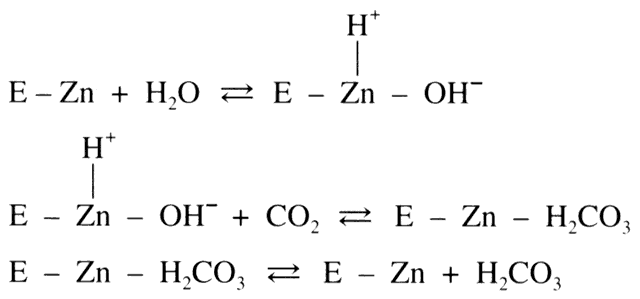1. Becker B: Decrease in intraocular pressure in man by a carbonic anhydrase inhibitor, Diamox: a
preliminary report. Am J Ophthalmol 37:13, 1954 2. Friedenwald JS: The formation of the intraocular fluid. Am J Ophthalmol 32:9, 1949 3. Kinsey VE: A unified concept of aqueous humor dynamics and the maintenance
of intraocular pressure: an elaboration of the secretion-diffusion
theory. Arch Ophthalmol 215, 1950 4. Wistrand PJ: Carbonic anhydrase in the anterior uvea of the rabbit. Acta Physiol Scand 24:144, 1951 5. Maren TH: The development of ideas concerning the role of carbonic anhydrase
in the secretion of aqueous humor: Relation to the treatment of
glaucoma. In Drance SM, Neufeld AH (eds): Glaucoma: Applied Pharmacology
in Medical Treatment, pp 325–355. Orlando, FL, Grune & Stratton, 1984 6. Maren TH: Carbonic anhydrase: chemistry, physiology and inhibition. Physiol Rev 47:595, 1967 7. Maren TH: The kinetics of HCO3- synthesis related to fluid secretion, pH control, and
CO2 elimination. Ann Rev Physiol 50:695, 1988 8. Kaunisto K, Parkkila S, Tammel T et al: Immunohistochemical localization of carbonic anhydrase isoenzymes in the
human male reproductive tract. Histochemistry 94:381, 1990 9. Hageman GS, Zhu XL, Waheed A, Sly WS: Localization of carbonic anhydrase IV in a specific capillary bed of the
human eye. Proc Natl Acad Sci USA 88:2716, 1991 10. Wistrand PJ, Schenholm M, Lönnerholm G: Carbonic anhydrase isoenzymes CA I and CA II in the human eye. Invest Ophthalmol Vis Sci 27:419, 1986 11. Maren TH: Current status of membrane-bound carbonic anhydrase. Ann NY Acad Sci 341:246, 1980 12. Wistrand PJ: The importance of carbonic anhydrase B and C for the unloading of CO2 by the human erythrocyte. Acta Physiol Scand 113:417, 1981 13. Maren TH, Wynns GC, Wistrand PJ: Chemical properties of carbonic anhydrase IV, the membrane-bound enzyme. Mol Pharmacol 44:901, 1993 14. Ashby W: Carbonic anhydrase in mammalian tissue. J Biol Chem 151:521, 1943 15. Brechue WF, Stager JM, Lukaski HC: Body water and electrolyte responses to acetazolamide in humans. J Appl Physiol 69:1397, 1990 16. Preisig PA, Toto RD, Alpern RJ: Carbonic anhydrase inhibitors. Renal Physiol 10:136, 1987 17. DuBose TD: Carbonic anhydrase-dependent bicarbonate transport in the kidney. Ann NY Acad Sci 429:528, 1984 18. Chapron DJ, Gomolin IH, Sweeney KR: Acetazolamide blood concentrations are excessive in the elderly: propensity
for acidosis and relationship to renal function. J Clin Pharmacol 29:348, 1989 19. Nadell J, Kalinsky H: The effects of the carbonic anhydrase inhibitor “6063” on electrolytes
and acid-base balance in two normal subjects and two patients
with respiratory acidosis. J Clin Invest 32:622, 1953 20. Benedikt O, Zirm M, Harnoncourt K: Relations between metabolic acidosis and intraocular pressure after inhibition
of carbonic anhydrase with acetazolamide. Graefes Arch Klin Ophthalmol 190:247, 1974 21. Zimmerman TJ, Garg LC, Vogh BP et al: The effect of acetazolamide on the movements of anions into the posterior
chamber of the dog eye. J Pharmacol Exp Ther 196:510, 1976 22. Becker B: The mechanism of the fall in intraocular pressure induced by the carbonic
anhydrase inhibitor, Diamox. Am J Ophthalmol 39:177, 1955 23. Krupin T, Oestrich CJ, Bass J et al: Acidosis, alkalosis, and aqueous humor dynamics in rabbits. Invest Ophthalmol Vis Sci 16:997, 1977 24. Langham ME, Lee PM: Action of Diamox and ammonium chloride on formation of aqueous humor. Br J Ophthalmol 41:65, 1957 25. Wistrand P, Maren TH: The effect of carbonic anhydrase inhibition on intraocular pressure of
rabbits with different blood CO2 equilibria. Am J Ophthalmol 50:291, 1960 26. Friedman Z, Krupin T, Becker B: Ocular and systemic effects of acetazolamide in nephrectomized rabbits. Invest Ophthalmol Vis Sci 23:209, 1982 27. Maren TH, Wadsworth BC: Blocking of renal effect of Diamox 2-acetylamino-1,3,4-thiadiazole-5-sulfonamide
by metabolic acidosis. Fed Proc 13:383, 1954 28. Bietti G, Virno M, Pecori-Giraldi J et al: Acetazolamide, metabolic acidosis, and intraocular pressure. Am J Ophthalmol 80:360, 1975 29. Stone RA, Zimmerman TJ, Shin DH et al: Low-dose methazolamide and intraocular pressure. Am J Ophthalmol 83:674, 1977 30. Dahlen K, Epstein DL, Grant M et al: A repeated dose-response study of methazolamide in glaucoma. Arch Ophthalmol 96:2214, 1978 31. Becker B: Carbonic anhydrase and the formation of aqueous humor: The Friedenwald
Memorial Lecture. Am J Ophthalmol 47:342, 1959 32. Bloom JN, Levene RZ, Thomas G et al: Fluorophotometry and the rate of aqueous flow in man: I. Instrumentation
and normal values. Arch Ophthalmol 94:435, 1976 33. Dailey RA, Brubaker RF, Bourne WM: The effects of timolol maleate and acetazolamide on the rate of aqueous
formation in normal human subjects. Am J Ophthalmol 93:232, 1982 34. McCannel CA, Heinrich SR, Brubaker RF: Acetazolamide but not timolol lowers aqueous humor flow in sleeping humans. Graefes Arch Clin Exp Ophthalmol 230:518, 1992 35. Friedland BR, Mallonee J, Anderson DR: Short-term dose response characteristics of acetazolamide in man. Arch Ophthalmol 95:1809, 1977 36. Lichter PR, Musch DC, Medzihradsky F et al: Intraocular pressure effects of carbonic anhydrase inhibitors in primary
open-angle glaucoma. Am J Ophthalmol 107:11, 1989 37. Kolker AE, Hetherington J: Carbonic anhydrase inhibitors. In Kolker AE, Hetherington
J (eds): Becker-Shaffer's Diagnosis and Therapy of
the Glaucomas, pp 384–392. St. Louis, CV Mosby, 1983 38. Garner LL, Carl EF, Ferwerda JR: Advantages of sustained-release therapy with acetazolamide in glaucoma. Am J Ophthalmol 55:323, 1963 39. Berson FG, Epstein DL, Grant WM et al: Acetazolamide dosage forms in the treatment of glaucoma. Arch Ophthalmol 98:1051, 1980 40. Joyce PW, Mills ICB, Richardson T et al: Equivalence of conventional and sustained release oral dosage formulations
of acetazolamide in primary open angle glaucoma. Br J Clin Pharm 27:597, 1989 41. Lehmann B, Linnér E, Wistrand PJ: The pharmacokinetics of acetazolamide in relation to its use in the treatment
of glaucoma and to its effects as an inhibitor of carbonic anhydrases. Adv Biosci 5:197, 1970 42. Linnér E, Wistrand P: The initial drop of the intraocular pressure following intravenous administration
of acetazolamide in man. Acta Ophthalmol 37:209, 1959 43. Becker B: Use of methazolamide (Neptazane) in the therapy of glaucoma, comparison
with acetazolamide (Diamox). Am J Ophthalmol 49:1307, 1960 44. Maren TH, Haywood JR, Chapman SK et al: The pharmacology of methazolamide in relation to the treatment of glaucoma. Invest Ophthalmol Vis Sci 16:730, 1977 45. Naimark A, Cherniack RM: Effect of dichlorphenamide on gas exchange and CSF acid-base state in chronic
respiratory failure. Can Med Assoc J 94:164, 1966 46. Kass MA, Kolker AE, Gordon M et al: Acetazolamide and urolithiasis. Ophthalmology 88:261, 1981 47. Shah A, Constant MA, Becker B: Urinary excretion of citrate in humans following administration of acetazolamide (Diamox). Arch Ophthalmol 59:536, 1958 48. Constant MA, Becker B: The effect of carbonic anhydrase inhibitors on urinary excretion of citrate
by humans. Am J Ophthalmol 49:929, 1960 49. Ellis PP: Urinary calculi with methazolamide therapy. Doc Ophthalmol 34:137, 1973 50. Shields MB, Simmons RJ: Urinary calculus during methazolamide therapy. Am J Ophthalmol 81:622, 1976 51. Spaeth GL: Potassium, acetazolamide, and intraocular pressure. Arch Ophthalmol 78:578, 1967 52. Leaf A, Schwartz WB, Relman AS: Oral administration of a potent carbonic anhydrase inhibitor (Diamox): I. Changes
in electrolyte and acid-base balance. N Engl J Med 250:759, 1954 53. Hanley T, Platts MM: Observations on the metabolic effects of the carbonic anhydrase inhibitor
Diamox: mode and rate of recovery from the drug's action. J Clin Invest 35:20, 1956 54. Epstein DL, Grant WM: Carbonic anhydrase inhibitor side effects: serum chemical analysis. Arch Ophthalmol 95:1378, 1977 55. Block ER, Rostand RA: Carbonic anhydrase inhibition in glaucoma: hazard or benefit for the chronic
lunger? Surv Ophthalmol 23:169, 1978 56. Chiesa A, Stretton TB, Massoud AAE et al: The effects of inhibition of carbonic anhydrase with dichlorphenamide on
ventilatory control at rest and on exercise in normal subjects. Clin Sci 37:689, 1969 57. Coudon WL, Block AJ: Acute respiratory failure precipitated by a carbonic anhydrase inhibitor. Chest 69:112, 1976 58. Goldberg MF: Sickled erythrocytes, hyphema, and secondary glaucoma: V. The effect of
vitamin C on erythrocyte sickling in aqueous humor. Ophthalmic Surg 10:70, 1979 59. Anderson CJ, Kaufman PL, Sturm RJ: Toxicity of combined therapy with carbonic anhydrase inhibitors and aspirin. Am J Ophthalmol 86:516, 1978 60. Hill JB: Salicylate intoxication. N Engl J Med 288:1110, 1973 61. Hill JB: Experimental salicylate poisoning: observations on the effects of altering
blood pH on tissue and plasma salicylate concentrations. Pediatrics 47:658, 1971 62. De Vincentiis M, Marmo F: Inhibition of the morphogenesis of the otoliths in the chick embryo in
the presence of carbonic anhydrase inhibitors. Experientia 24:818, 1968 63. Worsham F, Beckman EN, Mitchell EH: Sacrococcygeal teratoma in a neonate: association with maternal use of
acetazolamide. JAMA 240:251, 1978 64. Layton WM, Hallesy DW: Deformity of forelimb in rats: association with high doses of acetazolamide. Science 149:306, 1965 65. Maren TH: Teratology and carbonic anhydrase inhibition. Arch Ophthalmol 85:1, 1971 66. Rentiers PK, Johnston AC, Buskard N: Severe aplastic anemia as a complication of acetazolamide therapy. Can J Ophthalmol 5:337, 1970 67. Lubeck MJ: Aplastic anemia following acetazolamide therapy. Am J Ophthalmol 69:684, 1970 68. Wisch N, Fischbein FL, Siegel R et al: Aplastic anemia resulting from the use of carbonic anhydrase inhibitors. Am J Ophthalmol 75:130, 1973 69. Werblin TP, Pollack IP, Liss RA: Blood dyscrasias in patients using methazolamide (Neptazane) for glaucoma. Ophthalmology 87:350, 1980 70. Fraunfelder FT, Meyer SM, Bagby GC et al: Hematologic reactions to carbonic anhydrase inhibitors. Am J Ophthalmol 100:79, 1985 71. Ellis PP: Discussion. Ophthalmology 87:354, 1980 72. Mogk LG, Cyrlin MN: Blood dyscrasias and carbonic anhydrase inhibitors. Ophthalmology 95:768, 1988 73. Leopold IH, Eisenberg IJ, Yasuna J: Experiences with Diamox. Am J Ophthalmol 39:885, 1955 74. Gandham SB, Spaeth GL, DiLeonardo M et al: Methazolamide-induced skin eruptions. Arch Ophthalmol 111:370, 1993 75. Maren TH, Jankowska L, Sanyal G et al: The transcorneal permeability of sulfonamide carbonic anhydrase inhibitors
and their effect on aqueous humor secretion. Exp Eye Res 36:457, 1983 76. Stein A, Pinke RP, Glabb KT et al: The effect of topically administered carbonic anhydrase inhibitors on aqueous
humor dynamics in rabbits. Am J Ophthalmol 95:222, 1983 77. Lewis RA, Schoenwald RD, Barfknecht CF et al: Aminozolamide gel: a trial of topical carbonic anhydrase inhibitor in ocular
hypertension. Arch Ophthalmol 104:842, 1986 78. Kalina PH, Shetlar DJ, Lewis RA et al: 6-Amino-2-benzothiazole-sulfonamide: the effect of a topical carbonic anhydrase
inhibitor on aqueous humor formation in the normal human eye. Ophthalmology 95:772, 1988 79. Bar-Ilan A, Pessah NI, Maren TH: Ocular hypotensive activity and disposition of the topical carbonic anhydrase
inhibitor 6-hydroxy-benzo[b]thiophene-2-sulfonamide, L-650,719, in
the rabbit. J Ocul Pharmacol 5:99, 1989 80. Werner EB, Gerber DS, Yoder YJ: Effect of a topical carbonic anhydrase inhibitor, 6-hydroxybenzo[b]thiophene-2-sulfonamide, on intraocular pressure in normotensive
subjects. Can J Ophthalmol 22:316, 1987 81. Ponticello GS, Freedman MB, Habecker CN et al: Thienothiopyran-2-sulfonamides: a novel class of water-soluble carbonic
anhydrase inhibitors. J Med Chem 30:591, 1987 82. Baldwin JJ, Ponticello GS, Anderson PS et al: Letter to the editor: Thienothiopyran-2-sulfonamides: novel topically active
carbonic anhydrase inhibitors for the treatment of glaucoma. J Med Chem 32:2510, 1989 83. Maren TH, Bar-Ilan A, Conroy CW et al: Chemical and pharmacological properties of MK-927, a sulfonamide carbonic
anhydrase inhibitor that lowers intraocular pressure by the topical
route. Exp Eye Res 50:27, 1990 84. Sugrue MF, Gautheron P, Grove J et al: MK-927: A topically active ocular hypotensive carbonic anhydrase inhibitor. J Ocul Pharmacol 6:9, 1990 85. Wang RF, Serle JB, Podos SM, Sugrue MF: The effect of MK-927, a topical carbonic inhibitor, on IOP in glaucomatous
monkeys. Curr Eye Res 9:163, 1990 86. Lippa EA, Von Denffer HA, Hofmann HM et al: Local tolerance and activity of MK-927: a novel topical carbonic anhydrase
inhibitor. Arch Ophthalmol 106:1694, 1988 87. Bron AM, Lippa EA, Hofmann HM et al: MK-927: A topically effective carbonic anhydrase inhibitor in patients. Arch Ophthalmol 107:1143, 1989 88. Pfeiffer N, Hennekes R, Lippa EA et al: A single dose of the topical carbonic anhydrase inhibitor MK-927 decreases
IOP in patients. Br J Ophthalmol 74:405, 1990 89. Higginbotham EJ, Kass MA, Lippa EA et al: MK-927: A topical carbonic anhydrase inhibitor: dose response and duration
of action. Arch Ophthalmol 108:65, 1990 90. Serle JB, Lustgarten JS, Lippa EA et al: MK-927: A topical carbonic anhydrase inhibitor: dose response and reproducibility. Arch Ophthalmol 108:838, 1990 91. Tuulonen A, Hovding G, Gustad L et al: Multiple-dose dose-response curve of the topical carbonic anhydrase inhibitor
MK-927 (abstr). Invest Ophthalmol Vis Sci 30(suppl):24, 1989 92. Higginbotham E, Kao SF, Kass M et al: Once-daily and twice-daily treatment with the topical carbonic anhydrase
inhibitor MK-927 (abstr). Invest Ophthalmol Vis Sci 30(suppl):23, 1989 93. Serle JB, Lustgarten J, Lippa EA et al: Six week safety study of 2% MK-927 administered twice daily to ocular hypertensive
volunteers. J Ocul Pharmacol 8:1, 1992 94. Diestelhorst M, Bechetoille A, Lippa E et al: Comparative potencies of the topical carbonic anhydrase inhibitors MK-417 and
MK-927 (abstr). Invest Ophthalmol Vis Sci 30(suppl):23, 1989 95. Bron A, Lippa EA, Gunning F et al: Multiple-dose efficacy comparison of the two topical carbonic anhydrase
inhibitors sezolamide and MK-927. Arch Ophthalmol 109:50, 1991 96. Lippa E, Sherwood M, Laibovitz R et al: MK-417 vs. timolol comparative activity (abstr). Invest Ophthalmol Vis Sci 31(suppl):232, 1990 97. Biollaz J, Lippa EA, Buclin T et al: Stereoselective pharmacokinetics of
MK-927, a novel topical carbonic anhydrase inhibitor following ocular
administration. Eur J Clin Pharmacol 36(suppl):05.20, 1989 98. Buclin T, Biollaz J, Lippa EA et al: Absence of metabolic effects of the topical carbonic anhydrase inhibitors
MK-927 and sezolamide during two-week ocular administration to normal
subjects. Clin Pharmacol Ther 49:665, 1991 99. Baldwin JJ, Ponticello GS, Murcko et al: Three-dimensional structure of the carbonic anhydrase inhibitor complex
derived from human carbonic anhydrase II and the optical isomers of MK-927. Invest Ophthalmol Vis Sci 30(suppl):374, 1989 100. Sugrue MF, Mallorga P, Schwam H et al: A comparison of L-671,152 and MK-927, two topically effective ocular hypotensive
carbonic anhydrase inhibitors, in experimental animals. Curr Eye Res 9:607, 1990 101. Wang RF, Serle JB, Podos SM et al: The ocular hypotensive effect of the topical carbonic anhydrase inhibitor
L-671,152 in glaucomatous monkeys. Arch Ophthalmol 108:511, 1990 102. Sugrue MF, Funk H, Klotzbuecher C: Comparison of the topical carbonic anhydrase inhibitor L-671,152 and timolol
in glaucoma monkeys. Invest Ophthalmol Vis Sci 31(suppl):232, 1990 103. Wang RF, Serle JB, Podos SM et al: MK-507 (L-671,152), a topically active carbonic anhydrase inhibitor, reduces
aqueous humor production in monkeys. Arch Ophthalmol 109:1297, 1991 104. Hofmann HM, Feicht B, Brunner-Ferber F et al: MK-507 (L-671,152): lokale Vertäglichkeit und Wirksamkeit eines neven
lokalen Karboanhydrasehemmers bei gesunden Probanden. Fortsch Ophthalmol 88:513, 1991 105. Lippa EA, Laibovitz R, Clineschmidt C: L-671,152: A novel, active topical
carbonic anhydrase inhibitor in patients (abstr). Singapore, Glaucoma
Society International Congress of Ophthalmology, 1990 106. Lippa EA, Schuman JS, Higginbotham EJ et al: MK-507 versus sezolamide: comparative efficacy of two topically active
carbonic anhydrase inhibitors. Ophthalmology 98:308, 1991 107. Lippa EA: Topical carbonic anhydrase inhibitors. In Dodgson S (ed): The
Carbonic Anhydrases: Cellular Physiology and Molecular Genetics, pp 171–181. New
York, Plenum Press, 1991 108. Bourgeois H, Bron A, Lippa E et al: 2% L-671,152: Multiple-dose activity BID. Invest Ophthalmol Vis Sci 31(suppl):233, 1990 109. The MK-507 Clinical Study Group: Long-term glaucoma treatment with MK-507, Dorzolamide, a
topical carbonic anhydrase inhibitor. J Glaucoma 4:6, 1995 110. Lippa EA, Carlson LE, Ehinger B et al: Dose response and duration of action of dorzolamide, a topical carbonic
anhydrase inhibitor. Arch Ophthalmol 110:495, 1992 111. Lippa EA, Clineschmidt CM, Tipping RW et al: Dorzolamide hydrochloride: six-week, dose-response study of an active topical
carbonic anhydrase inhibitor. Invest Ophthalmol Vis Sci 34(suppl):931, 1993 112. Strahlman E, Tipping R, Vogel R et al: A double-masked, randomized 1-year study comparing dorzolamide (Trusopt), timolol, and
betaxolol. Arch Ophthalmol 113:1009, 1995 113. Wilkerson M, Cyrlin M, Lippa EA et al: Four-week safety and efficacy study of dorzolamide, a novel active topical
carbonic anhydrase inhibitor. Arch Ophthalmol 111:1343, 1993 114. Kass MA, Laibovitz RA, Lippa EA et al: Comparative activity of 3% MK-507, a topical CAI, with betaxolol (abstr). Invest Ophthalmol Vis Sci 32(suppl):989, 1991 115. Gunning FP, Greve EL, Bron AM et al: Two topical carbonic anhydrase inhibitors sezolamide and dorzolamide in
Gelrite vehicle: a multiple-dose efficacy study. Graefes Arch Clin Exp Ophthalmol 231:384, 1993 116. Nardin G, Lewis R, Lippa EA et al: Activity of the topical CAI MK-507 bid when added to timolol bid (abstr). Invest Ophthalmol Vis Sci 32(suppl):989, 1991 117. Serle JB, Podos SM, Lippa EA, Klotzbuecher CM: Compassionate case usage of dorzolamide (MK-507) in 16 glaucoma patients (abstr). Invest Ophthalmol Vis Sci 35(suppl):2177, 1994 118. Kitazawa Y, Azuma I, Araie M et al: Topical dorzolamide hydrochloride can be a substitute for oral carbonic
anhydrase inhibitors (abstr). Invest Ophthalmol Vis Sci 35(suppl):2177, 1994 |  H2CO3) in the ciliary epithelium as well as in other tissues in the body. Carbonic
acid (H2CO3) dissociates into bicarbonate (HCO3-) and hydrogen ion (H+ ). In the eye, HCO3- then passes into the posterior chamber along with
sodium ions (Na+ ). This movement of HCO3- and Na+ draws copious amounts of fluid along with it, thus producing aqueous humor. Although
this reaction proceeds in a reversible fashion in the absence
of a catalyst, the presence of the enzyme CA provides an enhancement
of the reaction rate by more than 100-fold.7
H2CO3) in the ciliary epithelium as well as in other tissues in the body. Carbonic
acid (H2CO3) dissociates into bicarbonate (HCO3-) and hydrogen ion (H+ ). In the eye, HCO3- then passes into the posterior chamber along with
sodium ions (Na+ ). This movement of HCO3- and Na+ draws copious amounts of fluid along with it, thus producing aqueous humor. Although
this reaction proceeds in a reversible fashion in the absence
of a catalyst, the presence of the enzyme CA provides an enhancement
of the reaction rate by more than 100-fold.7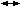 H2CO3). This reaction, when catalyzed by CA, proceeds to the right 13,000 times
as fast as the uncatalyzed reaction. As the erythrocyte proceeds from
tissue capillaries to pulmonary capillaries, H2CO3 is converted to H2O and CO2 (H2CO3
H2CO3). This reaction, when catalyzed by CA, proceeds to the right 13,000 times
as fast as the uncatalyzed reaction. As the erythrocyte proceeds from
tissue capillaries to pulmonary capillaries, H2CO3 is converted to H2O and CO2 (H2CO3 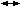 CO2 + H2O). In pulmonary capillaries, CO2 then diffuses across the red cell membrane into the plasma and then across
the alveolocapillary membranes into alveolar gas. Thus, CA plays
a critical role in the removal of the body's main metabolic byproduct, CO2.
CO2 + H2O). In pulmonary capillaries, CO2 then diffuses across the red cell membrane into the plasma and then across
the alveolocapillary membranes into alveolar gas. Thus, CA plays
a critical role in the removal of the body's main metabolic byproduct, CO2.