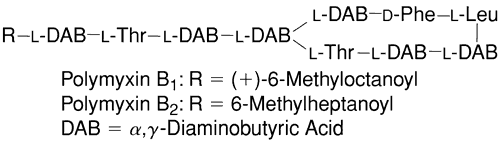FLUOROQUINOLONES
Chemistry, Ophthalmic Preparation, and Pharmacologic Action
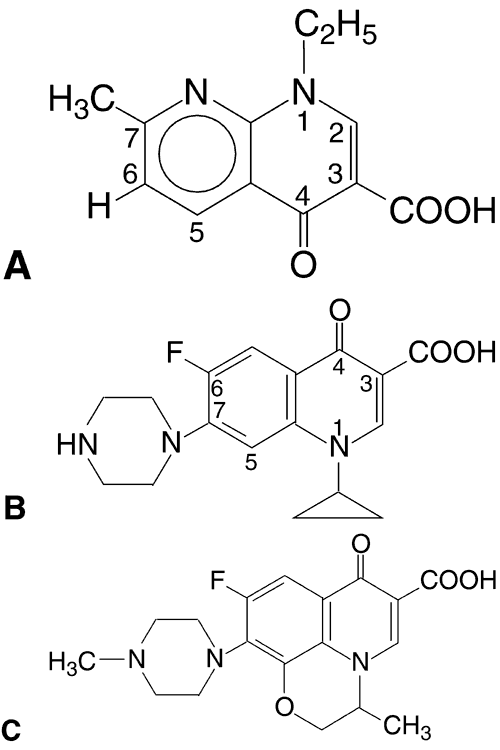
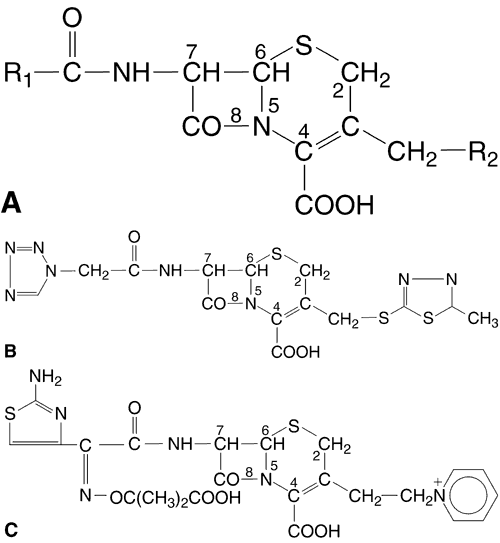
The fluoroquinolones, the newest class of agents to be developed, are based on the prototype, nalidixic acid (1,8-naphthyridine), which was synthesized in 1962.46
In the 1980s, the fluoroquinolones were created from nalidixic acid by adding a fluorine atom to position 6 of the molecule (Fig. 1). This addition widened the antibacterial spectrum of activity and resulted in decreased development of resistant organisms. Three fluoroquinolones available for ophthalmic use are ofloxacin (Ocuflox, Allergan, Inc, Irvine, CA), ciprofloxacin (Ciloxan, Alcon Laboratories, Inc, Fort Worth, TX), and norfloxacin (Chibroxin, Merck & Co, Inc, West Point, PA). They are formulated as 0.3% solutions and their structural formulas are shown in Figure 1.
|
The fluoroquinolones are bactericidal and work by inhibiting bacterial DNA gyrase (bacterial topoisomerase II), the enzyme responsible for maintaining the superficial twists in bacterial DNA.47 They provide broad-spectrum activity against gram-positive and gram-negative bacteria in vivo and in vitro. They have less predictable activity against anaerobes and streptococci.48
The fluoroquinolones are also available for systemic use, but clinical profiles of the ophthalmic and systemic formulations are distinctly different. For example, topical ophthalmic ofloxacin and ciprofloxacin are indicated for use in children as young as 1 year of age, whereas systemic formulations are approved only for use in older children and adults because of concerns about the potential risk of drug deposition in cartilage and arthropathy. In addition, the prevalence of fluoroquinolone-resistant bacteria is much higher among systemic pathogens than among the ocular pathogens commonly associated with conjunctivitis and keratitis. Moreover, although the use of systemic fluoroquinolones must be carefully considered to prevent further induction of resistant strains, this is of much less concern in ophthalmic use, because the strains of bacteria affected are much lower.49 Although resistance has been less of a concern in ophthalmic use, resistance to fluoroquinolone antibiotics has been increasing, and for this reason, their use is generally reserved for vision-threatening infections and infections not responding to conventional therapy.50
Clinical Experience and Ophthalmic Uses for the Individual Ophthalmic Preparations of the Fluoroquinolones
OFLOXACIN 0.3% SOLUTION
Ofloxacin has been tested against many previously established antibiotics in the treatment of external ocular infection and has been found to have a wide spectrum of activity.51–53 The Ofloxacin Study Group compared the effectiveness and safety of ofloxacin 0.3% with gentamicin 0.3% in the treatment of bacterial external ocular disease. Clinical improvement rates were 98% (51 of 52) in the ofloxacin group versus 92% (48 of 52) in the gentamicin group. Ofloxacin eradicated or controlled a similar proportion of cultured organisms as did gentamicin. There was no statistically significant difference in activity between the two drugs. The incidence of adverse effects attributable to ofloxacin treatment was 3.2% compared with 7.1% for gentamicin.54
The Ofloxacin Study Group also compared the efficacy of a 10-day course of topical ofloxacin to topical tobramycin in the treatment of external ocular infection. Initially, the clinical, microbiologic, and overall improvement rates were not statistically significantly different between the two groups. The ofloxacin-treated patients' examination signs and symptoms on days 3 to 5 were significantly more reduced than those of the tobramycin-treated patients.55,56
Ofloxacin has also been proven to be comparable with chloramphenicol in the treatment of external ocular infection57 while avoiding the risk of possibly fatal systemic complications that have been associated with topical chloramphenicol.
Ofloxacin has been compared with the traditional treatment regimen of a fortified aminoglycoside combined with a fortified cephalosporin in the treatment of bacterial keratitis. O'Brien and coworkers compared ofloxacin 0.3% monotherapy with tobramycin 1.5% plus cefazolin 10% therapy. The proportion of healed ulcers in both culture-positive treatment groups was similar (89% ofloxacin healed by 28 days and 86% of combination therapy healed by 28 days). Adverse effects were higher in the fortified antibiotics group; five of the six culture-positive patients who discontinued study medications were in the fortified antibiotics group.38 The Ofloxacin Study Group compared ofloxacin monotherapy to traditional dual therapy of fortified gentamicin 1.5% and cefuroxime 5%. This group found both treatments to be equally effective in the 49 culture-positive cases studied.51 Ofloxacin is more efficacious against methicillin-resistant S. aureus than ciprofloxacin is.58
Of the currently available fluoroquinolones, ofloxacin has the highest intrinsic solubility,59 is well tolerated because of its near-neutral pH (6.4), and has the highest rate of penetration into ocular tissues.60,61 This high rate of tissue penetration may also be significant in those cases in which physicians wish to use a topical antibiotic for prophylaxis in intraocular surgery.
CIPROFLOXACIN 0.3% SOLUTION
In two randomized multicenter studies, ciprofloxacin 0.3% was compared with a placebo and with tobramycin 0.3% in the treatment of culture-positive bacterial conjunctivitis. Ciprofloxacin was statistically significantly more effective than placebo with reduction or eradication of pathogens in 93.6% of ciprofloxacin-treated patients versus 59.5% of the placebo group. Ciprofloxacin and tobramycin were equally effective, with improved cultures in 94.5% and 91.9% of patients, respectively.62
Topical ciprofloxacin 0.3% has also been proven as safe and effective as tobramycin 0.3% and fusidic acid gel 1% in the treatment of bacterial conjunctivitis in a study of 257 pediatric patients. The investigators determined 87.0% of the ciprofloxacin-treated patients and 89.9% of the tobramycin-treated patients to be clinically cured after 7 days of treatment. No adverse effects occurred in either of the treatment groups.63–65
Monotherapy with ciprofloxacin 0.3% has been compared with combination therapy in the treatment of bacterial keratitis. Hyundik and coworkers studied 176 culture-positive cases of bacterial keratitis randomized to treatment with ciprofloxacin 0.3% or with tobramycin 0.3% and cefazolin 5%. There were no statistically significant differences between the treatment regimens in terms of overall clinical efficacy (91.5% versus 86.2%), time to cure, or reduction in signs and symptoms. The incidence of treatment failures was less with ciprofloxacin than with combination fortified therapy. Several other studies have examined the effectiveness of ciprofloxacin in treating ocular bacterial infections.66–73 There was a trend toward resistance ofS. pneumoniae to ciprofloxacin,39 but ciprofloxacin is more active against Streptococcus viridans, Pseudomonas aeruginosa, H. influenzae, and S. marcescens than ofloxacin is.58
Ciprofloxacin has shown potent in vitro activity against P. aeruginosa, including the aminoglycoside-resistant strains.47,74 The overall in vivo effectiveness of ciprofloxacin against pathogens, though high at approximately 92 percent, has declined in the past few years,75 and there is concern about the emergence of resistant strains of Staphylococcus and Pneumococcus species. There also exists an ointment form of ciprofloxacin 0.3% and one clinical trial has shown its effectiveness in treating bacterial keratitis.76
NORFLOXACIN 0.3% SOLUTION
Norfloxacin is the least potent of the topical fluoroquinolones.48,77,78 It has been shown to be effective in the treatment of bacterial conjunctivitis79 but not, with the exception of one small study, bacterial keratitis.80 In conjunctivitis, it is comparable in efficacy to tobramycin 0.3%81 and ciprofloxacin 0.3%.64 Norfloxacin has also been shown to be as effective as gentamicin 0.3% in the treatment of blepharitis and conjunctivitis.82 In the study by Miller and coworkers, norfloxacin suppressed or eliminated 89% of all organisms, based on pretreatment and posttreatment cultures. Norfloxacin has the highest rate of resistant bacteria among the fluoroquinolones based on in vitro testing with ocular isolates.59,83,84
Generalizations on the Group of Ophthalmic Fluoroquinolones
Multiple articles have supported the effectiveness of the fluoroquinolones in treating ocular bacterial infections.85–88
What are the advantages and disadvantages of the fluoroquinolones as compared with other, so-called fortified antibiotics? The aforementioned clinical studies conclusively show that ciprofloxacin and ofloxacin are statistically equal to fortified antibiotics in time to heal and cure rate of infectious corneal ulcers. However, other parameters should be analyzed. The acute management of bacterial corneal ulcers requires rapid access to therapy. In addition, the cost and toxicity of antibiotic therapy must be considered. Fortified antibiotics are not commercially available and must be prepared on request. The fluoroquinolones are superior with respect to accessibility, cost, and low toxicity. The fluoroquinolones perform at least as well as, and often better than, the aminoglycosides in the treatment of gram-negative corneal ulcers.
One of the main advantages of the fluoroquinolones is their high intrinsic solubility.60,89,90 Although ofloxacin is more soluble than ciprofloxacin, both achieve high intracorneal levels in patients with an intact epithelium.71 In a rabbit model with the epithelium intact, it was shown that ciprofloxacin achieves 10.47 μg/mL in the deep cornea; ofloxacin achieved 21.50 μg/mL.60 Ofloxacin is more lipophilic than ciprofloxacin, which may make it more effective in penetrating an intact epithelium.91 In most cases of bacterial keratitis, the epithelium is partially denuded and lipid solubility is not as important an issue because of the loss of the barrier function of the epithelium.92 However, when the more intact the overlying epithelium is, such as in the cases of suture abscesses after penetrating keratoplasty, the more likely ciprofloxacin and ofloxacin are to offer an advantage. The high stromal levels of ciprofloxacin and ofloxacin may explain the excellent clinical response by patients with ulcers despite intermediate or resistant laboratory susceptibility patterns in vitro.
The one species for which ciprofloxacin and ofloxacin are not of equal efficacy to fortified antibiotic solutions is streptococci. Neither in vitro nor clinically do they provide as good gram-positive coverage as cefazolin, vancomycin, or bacitracin. In a multicenter prospective, but nonblinded evaluation of ciprofloxacin 0.3% versus fortified antibiotics performed by Leibowitz,62 23.1% of S. pneumoniae corneal ulcers did not respond to ciprofloxacin. Based on previous in vitro data, case reports, and the two prospective evaluations, streptococci seem to be a weak point in the spectrum of activity of the fluoroquinolones. Therefore, any patient at increased risk of developing a S. pneumoniae infection should be treated with an antibiotic, such as bacitracin, vancomycin, or cefazolin, in addition to a fluoroquinolone. There is a known increased incidence of streptococcal infections in suture abscesses after penetrating keratoplasty and pseudophakic bullous keratopathy. S. pneumoniae is also commonly seen in corneal ulcers related to dacryocystitis and filtering bleb infections. Anaerobic streptococcal infections, such as those causing a crystalline keratopathy, are unlikely to respond to fluoroquinolone monotherapy. Finally, patients with hospital-acquired corneal ulcers, in which there is an increased risk of methicillin-resistant S. aureus, should be treated at least in part with fortified vancomycin drops.
Adverse Effects
The fluoroquinolones have low rates of adverse effects.93 That of ofloxacin (consisting primarily of transient local irritation) is less than 0.6%,38,54–57,94 and there has never been any report of corneal epithelial toxicity with ofloxacin.95 The Ofloxacin Study Group51 noted that fortified antibiotics showed drug toxicity to the ocular surface (defined as punctate corneal staining, papillary conjunctival reaction, or conjunctival fluoresecin staining) in 50.8% of patients receiving combination therapy versus only 10.2% of ofloxacin-treated patients.
As with ofloxacin, fewer patients treated with ciprofloxacin than treated with fortified antibiotics, reported ocular discomfort.39 The most commonly reported adverse effect from topical ciprofloxacin treatment is the formation of a white crystalline precipitate in approximately 17% of treated eyes.39,63–65 This results from precipitation of the drug (formulated at pH 4.5), which is poorly soluble at the near-neutral pH of the tear film. The relationship between this precipitate and antibacterial efficacy is unknown. The precipitate resolves spontaneously without sequelae after cessation of the medication. A few patients may also experience some local burning or irritation. There have been a few case reports of corneal precipitates with norfloxacin use in bacterial keratitis,96 but the overall incidence of adverse effects seems to be low.
Like the 0.3% solution, the ciprofloxacin ointment has also been shown to cause a white precipitate that resolves spontaneously without sequelae after discontinuing it. Other adverse effects from the ointment include burning, puntate epitheliopathy, blurred vision, and tearing.76
EXTEMPORANEOUSLY COMPOUNDED FORTIFIED ANTIBIOTICS
Extemporaneously compounded fortified antibiotic eyedrop preparations contain high concentrations that are usually prepared from products formulated for intravenous use. A typical treatment regimen might consist of a combination of a cephalosporin (e.g., 50 mg/mL cefazolin) for gram-positive bacteria coverage and an aminoglycoside (e.g., 13 mg/mL tobramycin or gentamicin) for gram-negative bacteria coverage. However, these agents are not compatible when combined in the same solution and must be formulated separately and administered from different bottles. Another common extemporaneously fortified antibiotic is vancomycin 50 mg/mL.97 Most need to be refrigerated after dispensing to the patient, because, being derived from intravenous products, they do not contain a preservative. Instillation of the two agents must be separated by intervals of several minutes or more (e.g., 15 minutes might be ideal) to prevent washout of the first agent by the second. The high concentrations used in these preparations exacerbate their epithelial toxic potential. This is a special concern for the aminoglycosides.
In the treatment of bacterial keratitis, fortified cefazolin-aminoglycoside preparations are as effective as the fluoroquinolones ofloxacin and ciprofloxacin, but are more difficult to obtain and use. Not all pharmacies are equipped to formulate extemporaneously compounded agents and not all pharmacists are familiar with the procedures.
There has been some debate in the literature as to the efficacy of using a collagen shield as a vehicle to absorb and deliver drugs. Advocates argue that collagen shields soak up antibiotics and continuously deliver them to the cornea for several hours, enabling higher concentrations to be delivered for longer periods of time. However, some studies have found that collagen shields are not more efficacious than using fortified antibiotics alone.98,99 According to several other studies, collagen shields are labor intensive yet as effective in treating bacterial keratitis, as frequent dosages of drops.100–104
AMINOGLYCOSIDES
Chemistry, Ophthalmic Preparation, and Pharmacologic Action
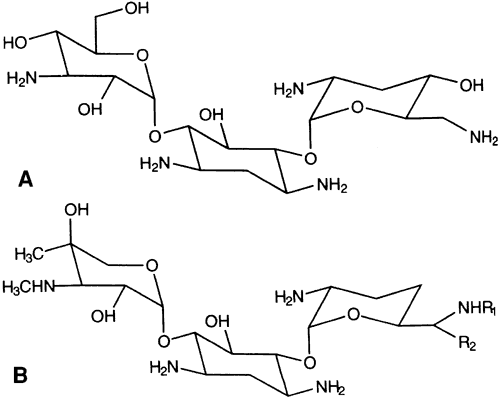
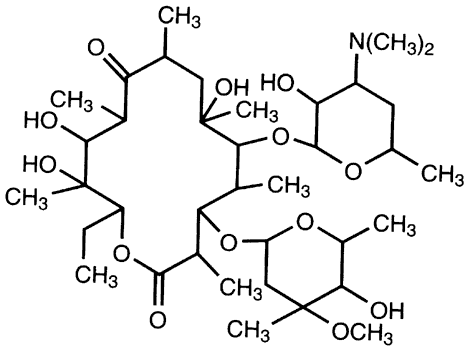
The two most commonly used aminoglycosides are tobramycin sulfate available as a 0.3% solution or ointment (AKTOB [Acorn, Inc, Buffalo Grove, IL], Defy, Tobrex [Alcon Laboratories, Fort Worth, TX]) and gentamicin sulfate (Fig. 2) available as a 0.3% solution (Garamycin, Genoptic [Allergan, Inc, Irvine, CA], Gentacidin, Gentak [Akorn, Inc, Buffalo Grove, IL], Ocu-mycin) and ointment (Garamycin, Genoptic). Neomycin [Bausch & Lomb Pharmaceutical, Inc, Tampa, FL] is also used, but is only available as a component of combination products, not as a single agent. The basic structure of aminoglycosides consists of two or more amino sugars connected by glycosidic bonds to a hexose nucleus. The individual characteristics of an aminoglycoside are determined by differences in the amino sugars attached to the nucleus.
The aminoglycosides cause bacterial cell death by irreversibly binding to 30S ribosomes and causing misreading of the genetic code and decreased or abnormal protein synthesis.105 Aminoglycosides are valued in the treatment of external ocular infections because they are active against aerobic gram-negative organisms, including Pseudomonas species, Proteus species, Klebsiella species, Escherichia coli, Salmonella species, Shigella species, S. marcescens, Haemophilus species and many gram-positive staphylococci.106 In vitro, tobramycin is three times as effective as gentamicin against Pseudomonas.107 The aminoglycosides have limited use as broad-spectrum agents because of resistance caused by aminoglycoside-modifying enzymes. This occurs at an unacceptably high frequency (29%–41%).108 Of particular concern is their lack of relative efficacy against S. epidermidis and S. pneumoniae.
Clinical Experience and Ophthalmic Uses
Gentamicin and tobramycin have been shown to be effective in the treatment of conjunctivitis, blepharoconjunctivitis, and bacterial keratitis.105,109–111 The commercially available concentrations are acceptable for the treatment of bacterial conjunctivitis, but the highly concentrated, fortified preparations are preferred for bacterial keratitis and are best used in conjunction with an antibiotic more active against gram-positive bacteria.
Adverse Effects
Gentamicin and tobramycin have been shown to be safe, but tobramycin may have fewer adverse effects.112 The most significant safety concern with aminoglycosides is corneal epithelial toxicity.108,113,114 This is especially so for neomycin and gentamicin.113 Lass et al. evaluated the concentration-dependent toxicities of neomycin, amikacin, gentamicin, and tobramycin using a rabbit epithelial cell culture model.113 Subfortified concentrations of neomycin and gentamicin significantly inhibited epithelial cell metabolism after 5 minutes of exposure; all the aminoglycosides significantly inhibited cell metabolism at all tested concentrations after 30 and 60 minutes of exposure. Fortified doses may cause a reversible punctate epithelial keratitis or pseudomembranous conjunctivitis.115,116 Several cases of conjunctival defects or necrosis have been reported with the use of fortified gentamicin,114,117 and at least one case of conjunctival necrosis has been attributed to fortified tobramycin use.114 Two cases of pseudomembranous conjunctivitis secondary to topical gentamicin have been reported: one case after use of commercial-strength gentamicin and one in response to fortified 1.36% gentamicin.118 Neomycin has a high rate of associated allergic reactions; in one study, 18.5% of 27 patients with chronic conjunctivitis had patch test sensitivity to neomycin.119 Other neomycin toxic manifestations are conjunctivitis, eyelid edema, punctate corneal erosions, and in high concentrations, reduced corneal sensation.120
BACITRACIN
Chemistry, Ophthalmic Preparation, and Pharmacologic Action
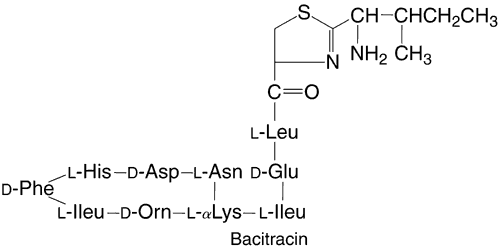
Bacitracin is a polypeptide antibiotic that contains a thiazolidine ring structure (Fig. 3). Bacitracin is bactericidal by binding to cell membranes121 and is commercially produced as a topical ophthalmic ointment (AK-Tracin, Akorn, Inc, Buffalo Grove, IL) or in combination with polymyxin B (AK-poly-bac [Akorn, Inc, Buffalo Grove, IL], Polysporin, Polytracin [Medical Ophthalmics, Tarpon Springs, FL]) or with polymyxin B and neomycin (AK-Spore [Akorn, Inc, Buffalo Grove, IL], Neosporin [Monarch Pharmaceuticals, Bristol, TN], Ocu-spor B). All these preparations contain bacitracin in a concentration of 500 U per gram of ointment. Unlike most of the other antibacterial agents discussed in this chapter, bacitracin is only available for topical use because of its systemic toxicity and poor solubility.
|
Clinical Experience and Ophthalmic Uses
Bacitracin is efficacious against most gram-positive organisms and select gram-negative organisms, including penicillinase-producing staphylococci, Neisseria species, Haemophilus species, and Actinomyces species. Bacitracin penetrates an intact cornea poorly, but its penetration may be increased by a corneal epithelial defect.122
Adverse Effects
Commercially available preparations and fortified dosages of bacitracin generally do not irritate the ocular surfaces. Hypersensitivity reactions, namely skin eruptions, have been reported. There is one report of an acute anaphylactic reaction associated with topical application of bacitracin.123
BETA-LACTAM ANTIBIOTICS
Chemistry, Ophthalmic Preparation, and Pharmacologic Action
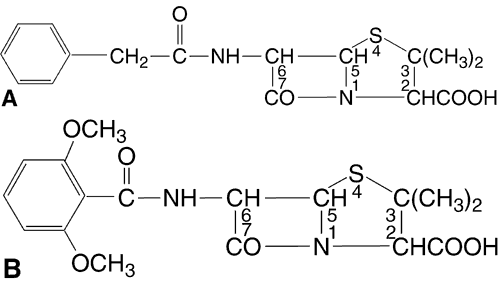
This class of antibiotics includes the penicillins and cephalosporins. Penicillins (Fig. 4) are composed of a thiazolidine ring connected to a beta-lactam ring to which a side chain is connected. The side chain is responsible for the individual characteristics of the penicillins. Like the penicillins, the cephalosporins (Fig. 5) contain a beta-lactam ring, are bactericidal, and inhibit cell wall synthesis. By preventing the synthesis of polysaccharides needed for bacterial cell wall structure, they cause bacterial death. They tend to be more active against gram-positive organisms, with increased gram-negative activity in the extended-spectrum penicillins and the second- and third-generation cephalosporins. Bacteria become resistant to penicillins by producing beta-lactamase; cephalosporins tend to be resistant to degradation by beta-lactamase. All methicillin-resistant S. aureus and enterococci are also resistant to cephalosporins. Approximately 10% of patients allergic to penicillin will also be allergic to cephalosporins.
|
|
Clinical Experience and Ophthalmic Uses
The beta-lactam antibiotics are not available in pharmaceutically manufactured topical ophthalmic preparations because of their poor stability. The most commonly used topical agent in this class is a first-generation cephalosporin, cefazolin 50 mg/mL, and is made from a parenteral preparation. As mentioned, cefazolin is used with a topical aminoglycoside in the treatment of bacterial keratitis. However, ceftazidime alone or in combination with an aminoglycoside or vancomycin has also been explored as an initial agent for topical therapy of bacterial keratitis.124 A third-generation cephalosporin, ceftazidime, was found to be as effective as cefazolin in treating rabbit corneal ulcers caused by S. aureus and S. pneumoniae and as effective as tobramycin against P. aeruginosa.125,126
The cefazolin-aminoglycoside combination has been proven to be equivalent to monotherapy with ofloxacin and ciprofloxacin in bacterial keratitis. Topical cefazolin may also have an important role in combination with fluoroquinolones. Bower and coworkers127 have predicted that 98.7% of their laboratory's ocular bacterial isolates would be susceptible to a fluoroquinolone-cefazolin combination versus 88.2%, 82.3%, and 80.4%, respectively, with ofloxacin, ciprofloxacin, and norfloxacin. Thus, in severe cases of bacterial keratitis, cefazolin may be a desirable addition to fluoroquinolone therapy while culture results are pending and may supplant the need for fortified aminoglycosides.128
Adverse Effects
The most common adverse effects are allergic reactions to the penicillins with some cross-allergenicity with the cephalosporins. Topical penicillin can result in anaphylaxis, and less significantly, there is a high incidence of contact allergic blepharitis.129 The cephalosporins have relatively few side effects,129 and approximately only 5% of patients manifest allergic reactions.
CHLORAMPHENICOL
Chemistry, Ophthalmic Preparation, and Pharmacologic Action
Chloramphenicol, a nitrobenzene derivative (Fig. 6), available as a 1% ointment or a 0.5% solution (AK-Chlor, Chlormycetin [Monarch Pharmaceuticals, Bristol, TN], Chloroptic [Allergan, Inc, Irvine, CA], Ocu-Chlor), was the first broad-spectrum antibiotic with gram-positive and gram-negative coverage. It has been widely used in ointment form for the treatment of external ocular infection.130
|
Chloramphenicol inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit. It is primarily bacteriostatic but may be bactericidal to some organisms (e.g., H. influenzae).
Clinical Experience and Ophthalmic Uses
Chloramphenicol has good antimicrobial activity against most gram-positive ocular isolates and limited gram-negative coverage. Chloramphenicol should not be used to treat infections in which gram-negative bacteria, especially Pseudomonas or Serratia species131 are suspected. Because it is usually bacteriostatic, not bacteriocidal, and because of its limited spectrum, chloramphenicol should not be used in vision-threatening circumstances.
In some studies, chloramphenicol has been shown to be as effective as ciprofloxacin, norfloxacin, and trimethoprim-polymyxin B in the treatment of bacterial conjunctivitis.79,132,133
Adverse Effects
Much has been written about a possible link between topical ophthalmic use of chloramphenicol and aplastic anemia. Oral chloramphenicol can affect the bone marrow in two ways; one is a dose-related, reversible bone marrow suppression and the other is an idiopathic, usually lethal effect. Topical chloramphenicol has been associated with dose-related and idiopathic bone marrow suppressions.134–140 Chloramphenicol should best not be used in patients who have a family history of drug-related bone-marrow failure.141,142 Concern about the risk of aplastic anemia and the development of more effective antibiotics have been sufficient to drastically reduce the use of chloramphenicol in the United States, although it is still widely used in other countries.
Burning may occur with topical instillation of chloramphenicol, but it is relatively nonirritating to ocular structures and allergic reactions are uncommon.
ERYTHROMYCIN
Chemistry, Ophthalmic Preparation, and Pharmacologic Action
Erythromycin 0.5% is a macrolide antibiotic (Fig. 7) that inhibits bacterial protein synthesis by irreversibly binding to the 50S ribosomal subunit.143 It is bacteriostatic in low concentrations but can be bactericidal in high concentrations. Other determinants of its bactericidal activity include organism susceptibility, growth rate of the bacteria, and pH.144 Erythromycin is available in ointment form (0.5%) for topical ophthalmic use (Ak-mycin, Ilotycin).
|
Clinical Experience and Ophthalmic Uses
Erythromycin is used as prophylaxis against neo-natal conjunctivitis caused by C. trachomatis andN. gonorrhoeae. It is also used to treat mild bacterial conjunctivitis. It has a broad spectrum of antibacterial activity145 and is well tolerated by the ocular surface, but many resistant strains have developed. For example, several strains of H. influenzae, one of the most common pathogens in pediatric conjunctivitis, are resistant to erythromycin. Therefore, its usefulness in the treatment of external ocular infections is limited.
Adverse Effects
Topical application of erythromycin is not usually irritating to ocular tissues.
POLYMYXIN AND COMBINATION PRODUCTS
Chemistry, Ophthalmic Preparation, and Pharmacologic Action
Many of the combination products currently available in the United States contain polymyxin B sulfate (Fig. 8). It provides efficacy against commonly encountered gram-negative pathogens such asH. influenzae. Polymyxin is a bactericidal polypeptide antibiotic that interferes with cell wall synthesis and forms false pores in bacterial cell membranes. It is less effective against Proteus, Providencia, Serratia, and Brucella species. It came into use in ophthalmology in the 1950s, when it was shown that polymyxin was effective in treating Pseudomonas corneal ulcers in rabbits.146 The effectiveness of this agent was then shown in treating human corneal ulcers infected with Pseudomonas species.147,148
|
Combinations of polymyxin B with neomycin and gramicidin or trimethoprim are available as solutions, and combinations with bacitracin, neomycin, and bacitracin, or oxytetracycline are available as ointments only. The ointments contain 10,000 U per gram of polymyxin B.
Two antibiotics commonly combined with poly-myxin are gramicidin and trimethoprim. Gramicidin alters bacterial cell wall permeability. Like bacitracin, it is only used topically because of systemic toxicity. Trimethoprim is a competitive inhibitor of bacterial dihydrofolate reductase, an enzyme that is necessary for purine synthesis.149 The other compounds that polymyxin B are combined with are addressed elsewhere in this chapter.
Clinical Experience and Ophthalmic Uses
All polymyxin combination products have shown efficacy against bacterial conjunctivitis,150,151 but no clinical studies of their use in the treatment of bacterial keratitis have been conducted. Clinical studies have shown that polymyxin B-trimethoprim andpolymyxin B-neomycin-gramicidin are as effectiveas each other152–153 and gentamicin sulfate,154 sodium sulfacetamide,154 and chloramphenicol138,150 in the treatment of bacterial conjunctivitis.
Adverse Effects
Those combination antibiotic products that do not contain the highly allergenic agent neomycin are commonly recommended in the literature.4,149,150 Polymyxin B rarely causes a hypersensitivity reaction. Chronic use of polymyxin B may result in toxic conjunctivitis.
SULFACETAMIDE
Chemistry, Ophthalmic Preparation, and Pharmacologic Action
Sulfacetamide (Fig. 9) 10% ointment (AK-Sulf, Cetamide, Sulamyd Sodium) and sulfacetamide 10% to 30% solutions (10% AK Sulf [Akorn, Inc, Buffalo Grove, IL], Bleph-10 [Allergan, Inc, Irvine, CA], Ophthacet, Ocusulf, Sulf-10 [CIBA Vision, Duluth, GA], Sulamyd Sodium, and Isopto Cetamide) act by preventing the incorporation of para-aminobenzoic acid into folic acid, thus inhibiting bacterial purine biosynthesis. Sulphonamides are bacteriostatic.
|
Clinical Experience and Ophthalmic Uses
Sulphonamides were used in the treatment of external ocular infections before the need to perform efficacy studies. It is difficult to find documentation of their value in the medical literature. In a study of 158 cases of culture-positive pediatric conjunctivitis, topical sulfacetamide was found to be equivalent in efficacy to trimethoprim-polymyxin B and gentamicin sulfate solutions.154 Like erythromycin, sulfacetamides have a broad spectrum of antibacterial activity, but many strains of resistant bacteria have developed. Sulfacetamide is still effective against H. influenzae but is ineffective against many staphylococcal isolates, S. marcescens, and P. aeruginosa, which makes it a poor choice as a first-line treatment for bacterial keratitis.155 It remains a drug of choice for the treatment of Nocardia species.156
Adverse Effects
Topical sulfacetamide is generally well tolerated. However, there is a small but significant risk of Stevens-Johnson syndrome associated with the use of topical sulfacetamide.157–159
TETRACYCLINES
Chemistry, Ophthalmic Preparation, and Pharmacologic Action
Tetracycline (Fig. 10) inhibits bacterial protein synthesis by binding to the 30S ribosome, and it is among the broadest spectrum agents available. For most organisms, tetracycline is bacteriostatic.
|
Clinical Experience and Ophthalmic Uses
Systemic and topical (ointment) tetracycline are used concurrently to treat C. trachomatis conjunctivitis. Tetracycline can also be used for prophylaxis against ophthalmia neonatorum from N. gonorrhoeae or chlamydial infections.160,161 In newborns developing ophthalmia neonatorum, coexisting oropharyngeal involvement usually requires more than topical drug use.
Adverse Effects
Adverse reactions from tetracyclines, including deposition in the teeth and bones, may be seen with both systemic use and topical application.
VANCOMYCIN
Chemistry, Ophthalmic Preparation, and Pharmacologic Action
Vancomycin is a complex bactericidal tricyclic glycopeptide (Fig. 11) that inhibits bacterial cell wall synthesis.162,163 It is active primarily against gram-positive bacteria, including methicillin-resistant S. aureus, S. epidermidis, and Enterococcus species.
|
Clinical Experience and Ophthalmic Uses
Topical vancomycin has been used successfully to treat chronic methicillin-resistant S. aureus in institutionalized patients.164 Vancomycin has not been tested against other antibiotics in the treatment of bacterial keratitis, but there are numerous reported cases of keratitis caused by resistant organisms that resolved with topical vancomycin therapy.165,166 Vancomycin 50 mg/mL has been tested against ciprofloxacin 0.3% in a rabbit model of methicillin-resistant S. aureus keratitis; ciprofloxacin was found to be more effective in that study.167 These results have not been verified in clinical studies. Vancomycin should be considered as a first-line therapy in severe cases of keratitis in patients at high risk for infection by methicillin-resistant organisms, such as healthcare workers or institutionalized patients.
Adverse Effects
Vancomycin is rarely used in routine ocular infections because it is not commercially available in a topical ophthalmic form. Its use should be limited because of the risk of the development of bacterial resistance. Topical application of vancomycin produces discomfort because of its low pH in solution. Adverse effects include conjunctival injection, chemosis, papillary conjunctivitis, and superficial punctate keratopathy. In rabbits, topical application was noted to retard corneal epithelial wound healing.168
SELECTING THE OPTIMAL OPHTHALMIC ANTIBIOTIC
The Goals of Treatment
In all bacterial infections, the goal of treatment is to rapidly eradicate the specific pathogen while minimizing adverse side effects and treatment costs. An ideal agent does not exist and the choice of the most appropriate agent must be based on a rational compromise. The nature of this compromise must be patient- and disease-specific.
CLINICAL JUDGMENT IN CHOOSING AN OPHTHALMIC ANTIBIOTIC
In uncomplicated acute bacterial conjunctivitis, the need for treatment efficacy, though still important to limit the rate of recurrence and spread to personal contacts, must be balanced by a strong assurance of treatment safety and comfort, particularly in children, and a reasonable concern for treatment cost. A further consideration is to limit the use of antibiotics needed for systemic infections so that widespread resistance does not occur. The preparation chosen is usually commercially available in a topical ophthalmic form and has a broad spectrum of efficacy against the most commonly implicated pathogens. The ophthalmologist and the patient can usually rest assured that they are dealing with a self-limited and usually benign process. In the treatment of acute bacterial conjunctivitis, no safety risks can be justified, and agents with known toxicity or a high incidence of hypersensitivity reactions should not be considered. However, in chronic or recurrent bacterial conjunctivitis, the ideal agent is the most effective broad-spectrum antibiotic available that the patient will tolerate, unless antibiotic susceptibility testing suggests that a specific narrow-spectrum agent may be more effective.
The sight-threatening nature of bacterial keratitis means that treatment speed and efficacy must take on more importance. Guidance in the choice of an antibiotic by knowing the specific organism and its sensitivity can be crucial to the outcome. The cost of treatment should only be taken into account if two or more treatment options are equal in efficacy and safety.153
BACTERIAL CONJUNCTIVITIS
Common Prescribing Practices
Physicians vary widely in their prescribing practices. Most cases of acute bacterial conjunctivitis are treated by nonophthalmologist physicians. Identification of the causative organism (e.g., by gram stain or culture) is rare. The use of a mild, broad-spectrum antibiotic preparation, such as one of the polymyxin B combination products (e.g., polymixin B-trimethoprim or polymixin B-zinc bacitracin) can be recommended. Sodium sulfacetamide is also commonly used, despite its bacteriostatic nature and limited spectrum because of its low cost and long clinical history. The use of chloramphenicol, because of the fear of aplastic anemia, and the use of neomycin, because of its relatively high rate of allergic reactions, are on the decline. Erythromycin cannot be recommended because of its lack of broad spectrum and the emergence of resistant H. influenzae. In more serious cases, a fluoroquinolone may be indicated because of its broad spectrum and efficacy against H. influenzae, as mentioned earlier, a common pathogen in pediatric conjunctivitis.
When bacterial conjunctivitis becomes chronic or recurrent culturing and sensitivity of the causative agent is recommended. In such cases, more aggressive treatment with a topical fluoroquinolone is recommended. Of the fluoroquinolones, ofloxacin followed by ciprofloxacin has the best coverage, and the lowest incidence of resistance. Their excellent tissue penetration capabilities will allow them to eradicate any bacteria that have breached the corneal epithelium and may also help reduce self-reinfection from pathogens sequestered in the meibomian orifices or lacrimal canaliculi.
BACTERIAL KERATITIS
Common Prescribing Practices
The medical literature documents the value of fortified antibiotics and the commercially available topical fluoroquinolone preparations in the treatment of bacterial keratitis. In a 1996 survey of 124 ophthalmologists from Florida, New York, and Illinois,169 82% reported that they would use a fluoroquinolone for initial treatment of less severe corneal ulcers, and 6% said that they would use fortified antibiotics. For more severe ulcers, prescribing practices shifted slightly toward fortified antibiotics, with 62% reporting that they would use fluoroquinolones for these cases and 23% stating that they would use fortified antibiotics. One common combination of extemporaneously compounded fortified antibiotics is cefazolin 10% and tobramycin 1.5% administered as separate solutions. Vancomycin 50 mg/mL should be substituted for cefazolin for those cases in which antibiotic susceptibility testing suggests that it will be the most effective agent or there is a significant risk of methicillin-resistant S. aureus. In vitro susceptibility testing has shown that the use of a fluoroquinolone in combination with fortified cefazolin is effective against more ocular isolates than ofloxacin, ciprofloxacin, or norfloxacin used alone. Therefore, fortified tobramycin may be replaced with commercially available ofloxacin or ciprofloxacin.127 It is not known whether the precipitation of ciprofloxacin has any detrimental effect on efficacy. Norfloxacin is the least effective of the available fluoroquinolones and has not been shown effective in the treatment of bacterial keratitis.