HOMOCYSTINURIA
Homocystinuria is an inborn error of metabolism of the sulfur-containing amino acids. Carson and Neill first detected homocystinuria using urine chromatography while systematically searching for metabolic abnormalities in mentally retarded institutionalized persons in Northern Ireland.1 Simultaneously and independently, Gerritsen and associates in Madison, Wisconsin, found the same metabolic defect in an infant with mental retardation and failure to thrive.2
Homocystinuria is an autosomal recessive disorder caused by a deficiency of the enzyme cystathionine beta-synthase, an intermediate in the degradation of homocysteine to cystine.3 The resulting blockage in this biochemical pathway causes accumulation of homocysteine precursors, homocystine and methionine, with increased concentrations of these amino acids in blood and urine. The genetic mutation has been mapped to chromosome 21 (21q22.3).
Four organ systems show major involvement in patients with homocystinuria: the skeletal, central nervous, and vascular systems and the eye . Excessive height and long thin extremities are common skeletal findings in the homocystinuric patient. These findings are similar to those of the Marfan syndrome. The most consistent skeletal abnormality is osteoporosis, which may lead to vertebral collapse.4 Mental retardation occurs in approximately 50% of cases and may be progressive. Thrombotic vascular occlusions constitute the main threat to survival in patients with homocystinuria. Large and small arteries and veins may be affected. Therefore, premature deaths from myocardial infarctions, pulmonary emboli, and cerebrovascular accidents are not uncommon.
Anesthesia may present significant risks to the homocystinuric patient.5–7 Mudd and colleagues documented 25 postoperative thromboembolic events in 586 homocystinuric patients undergoing surgical procedures, 6 of them fatal.8 Medications that may predispose these patients to a hypercoagulable state, such as oral contraceptives, should also be avoided.9
Ectopia lentis is the ocular hallmark of homocystinuria and can be detected in approximately 90% of patients.10,11 Ectopia lentis is an acquired and progressive abnormality in this disorder. The dislocation is bilateral, with the lens usually migrating either inferiorly or inferonasally. The causes of ectopia lentis are summarized in Table 2.
TABLE TWO. Differential Diagnosis of Ectopia Lentis
Genetic
Without systemic manifestation
Simple ectopia lentis
Congenital (recessive and dominant varieties reported)
Delayed onset (recessive and dominant varieties reported)
Ectopia lentis et pupillae (recessive)
Megalocornea (most commonly X-linked; dislocation of the lens a rare complication)
With systemic manifestation
Marfan syndrome
Homocystinuria
Weill-Marchesani syndrome
Hyperlysinemia
Sulfite oxidase deficiency
Nongenetic
Trauma
Leutic
(Modified from Maumenee IH: The eye in the Marfan syndrome. Trans Am Ophthalmol Soc 79:684, 1981)
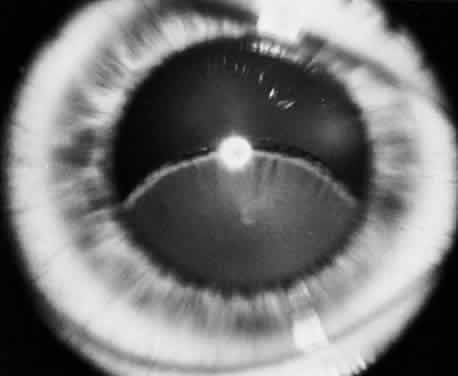
The lens in homocystinuria is much more mobile than in the Marfan syndrome. This may be related to the clinical observation of progressive irregularity of the zonular fibers and the appearance of a fringe of white zonular remnants at the equator of the lens and on the surface of the ciliary body (Fig. 1).12,13 Henkind and Ashton first reported histopathologically the ocular findings in four eyes of three homocystinuric patients. They found the zonular fibers to be deficient adjacent to the lens. These zonules had recoiled to the surface of the ciliary body and were matted and retracted into a feltwork that fused with a greatly thickened basement membrane of the nonpigmented epithelium. The greatly thickened basement membrane overlying the ciliary body in homocystinuria has subsequently been shown by electron microscopy to be composed of degenerate zonular material.14 In addition, Ramsey and coworkers noted that the degree of zonular abnormality was related to age: the younger the patient, the more normal-appearing zonular fragments composed of oriented filaments that could be identified.14 The zonular fibers are composed of glycoproteins with a high concentration of cysteine, which may explain their susceptibility to abnormal function in homocystinuria.15,16
|
Myopia is another common ocular finding and may precede the detection of ectopia lentis by several years.17 The development of progressive lenticular myopia is often the first sign of a lens dislocation. Retinal detachment is usually a complication of lens surgery, although it may occur spontaneously.11 Other ocular findings associated with homocystinuria are listed in Table 3.
TABLE THREE. Ocular Findings Associated With Homocystinuria and the Frequency
of Their Occurrence
| Finding | Frequency |
| Dislocation of lenses | Almost all cases |
| Myopia | Almost all cases |
| Fringe of zonules on dislocated lens | Most cases |
| Peripheral cystoid retinal degeneration | Usual |
| Optic atrophy | Frequent |
| Secondary glaucoma | Frequent |
| White deposits on the ciliary processes and zonular fibers | Uncertain |
| Congenital cataract | Rare |
| Central retinal artery occlusion | Rare |
| Aniridia | Rare |
| Coloboma of choroid and optic disc | Rare |
| Coloboma of iris | Rare |
| Microcornea | Rare |
| Microphthalmos | Rare |
One treatment of homocystinuria to correct the metabolic defect has involved a low-methionine and high-cystine diet, which has not been uniformly successful.18–22 This diet has not only failed to normalize completely the abnormal biochemical findings, but it is difficult to prescribe (all eggs, meat, and cow's milk are forbidden). When this diet is prescribed to infants, failure to gain weight may be a serious complication.23
Another approach to therapy is supplementation with coenzymes. Pyridoxine (vitamin B6) is the coenzyme necessary to activate cystathionine beta-synthetase.24 Barber and Spaeth were the first to demonstrate that certain homocystinuric patients respond biochemically to oral pyridoxine with lowered plasma concentrations of homocystine and methionine.25 A different mutation in the cystathionine beta-synthase gene is responsible for the observed difference in the individual response to pyridoxine.26,27 Homocystinurics who are B6-responsive have a less devastating clinical course, with a higher average IQ and less frequent major clinical abnormalities. In addition, B6-responsive individuals may show a decrease in the incidence of clinical abnormalities when treated with pyridoxine.8,28
Early identification of patients with homocystinuria is essential so that early and effective treatment can be provided. Spaeth and Barber first described a sodium-nitroprusside test to screen for homocystine in the urine.29 Because the test results are positive in many conditions in which sulfur-containing metabolites are excreted (e.g., cystinuria), urine chromatography or high-voltage electrophoresis is necessary for a definitive diagnosis. Some B6-responsive cases can be missed with current screening protocols.8,30
ALBINISM
Albinism comprises a heterogeneous group of clinical syndromes exhibiting hypomelanosis based on heritable metabolic defects of the melanin pigment system. All types of albinism are characterized by foveal hypoplasia, nystagmus, photophobia, and decreased visual acuity in addition to absent or decreased melanotic pigment in skin, hair, and eyes (oculocutaneous albinism [OCA]) or in the eye alone (ocular albinism).31 The term albinoidism refers to hypomelanotic disorders in which the patients do not have nystagmus, decreased visual acuity, or foveal hypoplasia. Eleven disorders have been identified with clinical features of oculocutaneous albinism and four with features of ocular albinism (Table 4). The ocular findings in oculocutaneous albinism are listed in Table 5 (Figs. 2 and 3).
TABLE FOUR. Classification of Inherited Hypopigmented Conditions With Ocular
Involvement
Oculocutaneous albinism
Hair bulb tyrosine test negative
Tyrosinase-negative (AR)
Yellow-mutant (AR)
Hair bulb tyrosine test positive
Tyrosinase-positive (AR)
Hermansky-Pudlak syndrome (AR)
Chédiak-Higashi syndrome (AR)
Cross syndrome (AR)
Rufous (AR)
Brown (AR)
Autosomal dominant
Black locks: congenital sensorineural deafness (AD)
Hair bulb tyrosine test questionable
Ocular albinism
Nettleship-Falls X-linked
Forsius-Eriksson X-linked
Autosomal recessive
Lentigines-deafness (AD)
AR, autosomal recessive; AD, autosomal dominant
TABLE FIVE. Ocular Signs in Oculocutaneous Albinism
Subnormal visual acuity (20/80 to 20/400)
Nystagmus
Extremes in refractive error
Strabismus
Iris transillumination
Macular hypoplasia
Visible choroidal vasculature
|
Three forms of OCA have been identified, each mapped to a different genetic locus. OCA type I represents tyrosinase-negative albinism and has been mapped to chromosome 11 (11q14-q21). There is a deficiency in the catalytic activity of tyrosinase, which is involved in at least three steps in the melanin biosynthetic pathway.32,33 In OCA type IA, the affected homozygote is “dead white” at birth, with no discernible pigment. In the yellow mutant form of OCA (type IB), some pigmentation of the skin forms and the hair develops a yellow coloration.
Tyrosinase-positive OCA (type II) has been mapped to chromosome 15 (15q11.2-q12) and is the most prevalent type of albinism in the world.34 The mutated protein is a transmembrane polypeptide that may play a role in transporting small molecules such as tyrosine, a precursor of melanin.35,36 Affected individuals show some degree of pigmentation and have less profound clinical manifestations than those with OCA type I.
In OCA type III (brown oculocutaneous albinism), a mutation in the tyrosinase-related protein-1 affects its interaction with tyrosinase, resulting in dysregulation of tyrosinase activity. This promotes the synthesis of brown melanin instead of black melanin.37 The mutation has been localized to chromosome 9 (9p23). Affected individuals have brown hair and skin and are capable of developing a faint tan with sun exposure.38
Individuals who are homozygous for the genetic mutations responsible for OCA have an abnormal proportion of fibers from the ganglion cells of the temporal retina that decussate to the contralateral cerebral hemisphere; this can be demonstrated by monocular visual evoked potential asymmetry.39,40 This may play a role in the high incidence of subnormal binocular vision and strabismus in albinos. This abnormality can be detected early in life and may be helpful in making the diagnosis of OCA during the neonatal period.41
All patients with OCA should be questioned about a history of bleeding because of the association of albinism with a hemorrhagic diathesis, the Hermansky-Pudlak syndrome.42 The hemostatic defect in the Hermansky-Pudlak syndrome is typically mild; the most common manifestations are gingival bleeding, epistaxis, and easy bruisability.43 More serious bleeding episodes have followed surgical procedures, especially when aspirin was used to control postoperative discomfort.44 The Hermansky-Pudlak syndrome is the most common form of albinism seen in Puerto Rico. A deficiency of platelet dense bodies, as observed by electron microscopy, is the most reliable method of diagnosing this syndrome.45
In affected persons with ocular albinism, the cutaneous pigmentary dilution is much less noticeable than is the ocular involvement. The skin pigmentation in these patients typically falls within the normal range. However, when these affected persons are compared with their nonaffected siblings, they typically have a lighter complexion.46 The ocular findings in ocular albinism are similar to those in OCA. However, in patients with ocular albinism, the pigmentary dilution of the eye may be subtle. In whites, there is usually iris transillumination. In blacks, the iris often does not transilluminate and the retina is usually moderately pigmented.47 Regardless of the pigmentation of affected patients, all have foveal hypoplasia.
In the Nettleship-Falls form of X-linked ocular albinism, there is an inborn error of metabolism affecting the pigment cells. This defect has been localized to the Xp22.3 region. Histopathologic examination of the clinically normal skin in affected males and carrier females has revealed the presence of macromelanosomes.48 Female carriers often show a mud-spattered appearance of the fundus with hy-perpigmented streaks as well as iris transillumination defects. These clinical signs, in addition to skin biopsy, can be used to help in genetic counseling for families at risk.49,50
TYROSINEMIA
There are five clinical syndromes in which elevated serum and urinary tyrosine levels and their metabolites can be detected: (1) tyrosinemia secondary to systemic disease (liver damage, pernicious anemia, vitamin C deficiency); (2) neonatal tyrosinemia, a transient benign process resulting from immature parahydroxyphenylpyruvate acid oxidase producing high serum tyrosine levels in premature infants; (3) tyrosinemia associated with hepatorenal disease (hereditary tyrosinemia, tyrosinemia type I, which is a rapidly fatal disease of infancy), methionemia, aminoaciduria, and glycosuria; (4) tyrosinemia associated with ocular but not hepatorenal disease (essential tyrosinemia, tyrosinemia type II); and (5) tyrosinemia without hepatorenal or ocular disease.
Type I tyrosinemia is caused by a defect in fumarylacetoacetate hydrolase (chromosome 15q23-q25), the last enzyme in the tyrosine catabolism pathway.51 An accumulation of succinylacetone occurs that reacts with other amino acids and proteins. Patients who survive beyond infancy are at high risk for the development of hepatocellular carcinoma; this has been prevented in some children who have undergone neonatal liver transplantation.52
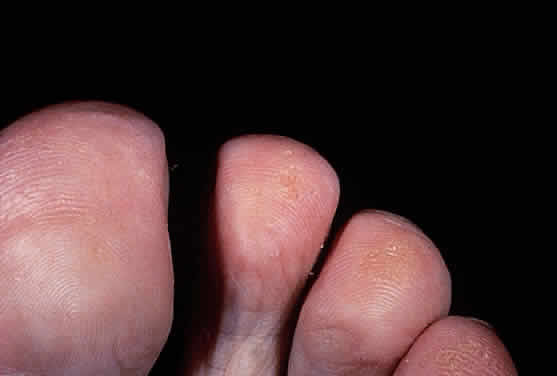
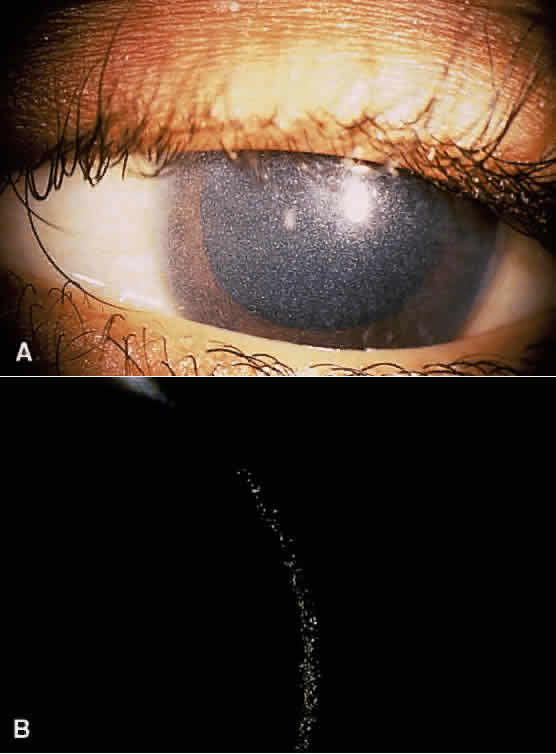
Tyrosinemia type II (Richner-Hanhart syndrome) is a rare congenital error of metabolism characterized by a triad of dendriform keratitis, hyperkeratotic lesions of the palms and soles, and mental retardation (Figs. 4 and 5).53–59 Many of the early reported cases of this disease were in patients who were the product of consanguineous marriages, suggesting a possible autosomal recessive inheritance.55,58–61
|
|
The diagnosis of tyrosinemia type II may be confirmed by amino acid analysis of the blood and urine, which shows an increase of tyrosine and its metabolites only. The enzymatic basis of the syndrome is a defect in soluble hepatic cytosol tyrosine aminotransferase, whose gene has been mapped to chromosome 16 (16q22.1-q22.3).55,62,63
Ocular symptoms of photophobia and lacrimation in both eyes usually appear during the first few months of life. The affected patient has bilateral subepithelial central corneal ulcers with herpetiform dendritic branching, which may develop with time into round whitish opacities with superficial new vessel formation.59 The corneal ulceration probably results from accumulation of intracellular crystals, presumably tyrosine. These crystals have been shown to enlarge, lacerate and rupture the cell, attract lysosomal enzymes, and trigger the inflammatory cycles.54,64
A low-tyrosine, low-phenylalanine diet has been used successfully in several patients with tyrosinemia type II, with rapid resolution of the clinical symptoms and signs.55,58,65–68 The keratitis found in tyrosinemia type II can be distinguished from herpes simplex keratitis by its morphologic appearance, bilateral presentation, lack of response to antiviral therapy, and associated systemic findings.53 Therefore, an infant or young child with bilateral dendritic keratitis should undergo serum and urinary evaluation for elevated levels of tyrosine and its metabolic byproducts.
Tyrosinemia type III is due to a defect in the enzyme 4-hydroxyphenylpyruvate dioxygenase, which has been mapped to chromosome 12 (12q24). Liver biopsy in affected individuals has been histologically normal, and mental retardation may be present.69
CYSTINOSIS
Cystinosis is an autosomal recessively inherited disorder of amino acid metabolism characterized by the deposition of cystine crystals in various tissues such as the eye, bone marrow, lymph nodes, and internal organs.70 Cystinosis is a lysosomal storage disorder that results from impaired transport of the disulfide amino acid cystine from cellular lysosomes. It differs from other lysosomal diseases because the main enzyme function of lysosomes, acid hydrolysis, does not play a role in cystine deposition. Plasma levels of cystine are below saturation level, whereas intracellular concentrations are elevated in polymorphonuclear leukocytes, macrophages, and fibroblasts, further indicating the cellular nature of the defect responsible for this disease.
Three clinical types of cystinosis have been described, all of which have ocular involvement (Table 6).71 The infantile, or nephropathic, variety is the most severe form of cystinosis.72 The gene has been mapped to the short arm of chromosome 17 (17p13).73 During early childhood, affected patients develop polyuria and polydipsia as a result of impaired renal tubular water reabsorption. This is followed by growth retardation, renal rickets, metabolic acidosis, progressive renal failure, and, ultimately, death from uremia before puberty.
TABLE SIX. Some Distinguishing Characterisitics of the Three Major Types
of Cystinosis
| Nephropathic (Infantile) | Adolescent | Benign | |
| General Symptoms | |||
| Onset of symptoms | 6–18 months | 18 months—17 years | No symptoms |
| Growth | Impaired | Variable | Normal |
| Skin pigmentation | Usually fair | Variable | Normal |
| Rickets | Present | Variable | Absent |
| Bone marrow cystine crystals | Present | Present | Present |
| Ocular | |||
| Retinopathy | Present | Variable | Absent |
| Crystalline deposits in cornea and conjunctiva | Present | Present | Present |
| Photophobia | Usuallypresent | Variable | Absent |
| Renal | |||
| Tubular dysfunction (Fanconi syndrome) | Present | Often incomplete | Absent |
| Glomerular failure | Present | Present at a later age than in infantile type | Absent |
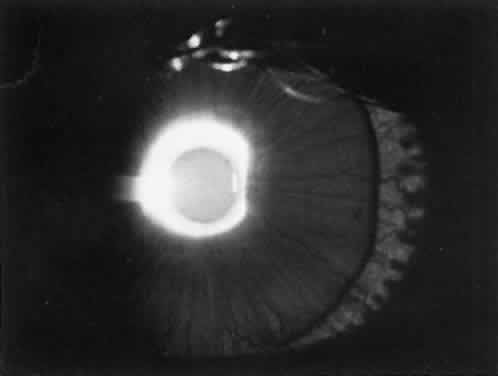
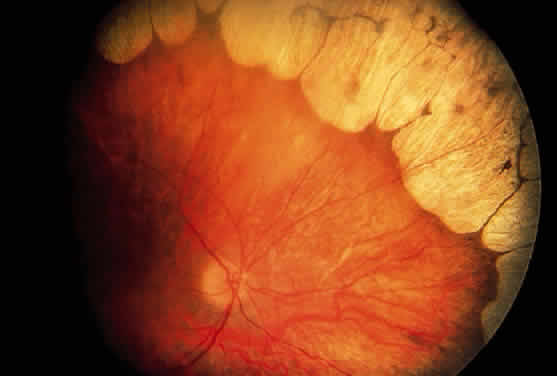
Children with nephropathic cystinosis usually develop severe photophobia within the first few years of life. Slit-lamp examination discloses diffuse scintillating, tinsel-like crystals of the cornea, conjunctiva, and iris (Fig. 6).74–76 Initially, the iridescent crystals occupy the full stromal thickness only in the periphery, while centrally the anterior half to two thirds of the stroma is involved. In patients with longstanding disease, thinning and focal breaks in Bowman's membrane may be present and contribute to the development of severe photophobia.77 Corneal sensitivity may be reduced.78
|
The essential fundus abnormality is a patchy depigmentation of the periphery and a finer “salt-and-pepper” disturbance at the posterior pole.72,79 Crystal deposition may also be seen (Fig. 7). Despite the extensive tissue infiltration of cystine crystals, there is no significant visual disturbance.
|
Cysteamine is a cystine-depleting agent used in the treatment of infantile nephropathic cystinosis. When given systemically, it has proven to be successful in retarding glomerular deterioration and enhancing growth, but it does not prevent the development of corneal crystal formation.70,73,80–82 Topical cysteamine drops have been shown not only to provide primary prevention of corneal crystal deposition but may also reverse the corneal complications in older patients.83,84
Adolescent cystinosis is a milder form of the disease, appearing in the first or second decade of life.85,86 This type of cystinosis may or may not include rickets, renal failure, photophobia, or retinopathy. All patients have shown the crystalline material in the cornea, conjunctiva, and reticuloendothelial system.
A benign adult form of cystinosis was first described by Cogan and coworkers in 1957.87 The primary clinical distinction between the benign and the nephropathic variants is failure of the former patients to show either retinopathy or renal dysfunction (see Table 6).88 Although affected patients may have mild photophobia, they are frequently diagnosed by routine ophthalmologic examination when the typical corneal crystals are noted.89,90
GYRATE ATROPHY
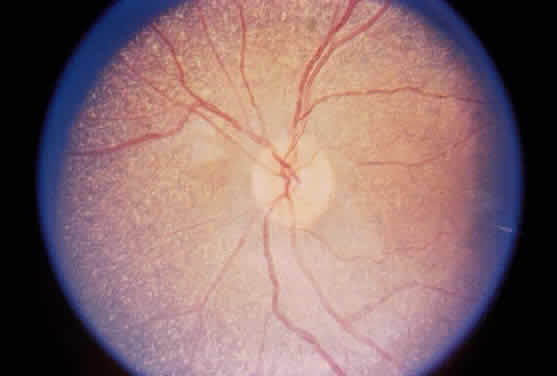
Gyrate atrophy of the choroid and retina is a rare autosomal recessively inherited, progressive, metabolic chorioretinal dystrophy beginning in childhood.91 It is caused by a deficiency in the mitochondrial matrix enzyme ornithine aminotransferase (OATase), resulting in hyperornithinemia.92–100 The genetic mutation has been mapped to chromosome 10 (10q26).101
Aside from visual impairment, patients with gyrate atrophy are for the most part asymptomatic. Mild to moderate diffuse slowing on electroencephalography has been reported in fewer than one third of affected patients.102–104 However, seizures have not been documented with increased frequency, and the majority of patients have normal intelligence.103,104 Liver biopsies in patients with gyrate atrophy have demonstrated nonspecific morphologic abnormalities of the mitochondria, with elongation, branching, and segmentation.105 The functional significance of these mitochondrial abnormalities is not known. However, these abnormalities are believed to be a direct result of hyperornithinemia because similar mitochondrial changes have been produced in rats maintained on high-ornithine diets.106
Tubular aggregates have been identified in type 2 skeletal muscle fibers in patients with gyrate atrophy.103–109 These aggregates are not specific for gyrate atrophy and have been identified in disorders such as periodic paralysis, hyperthyroidism, porphyria cutanea tarda, myasthenia gravis, myotonic dystrophy, postviral infections, and alcoholism.91,110 The disease progresses to almost complete loss of type 2 fibers, but the progression of muscle changes is slower than the progression of the ocular disease.
The major clinical problem in patients with gyrate atrophy is a slowly progressive loss of vision leading to blindness, usually by the fourth decade of life (Table 7).102,111–113 Myopia or decreased night vision is the earliest symptom, usually noted before the end of the first decade of life. Constriction of the visual field is obvious by the second decade. By age 40, most patients show visual fields smaller than 10°.112 Posterior subcapsular lens changes develop in late adolescence. After cataract surgery, there is generally a marked improvement in visual acuity, especially in younger patients.
TABLE SEVEN. Ocular Manifestations of Gyrate Atrophy
Progressive loss of vision
High myopia and marked astigmatism
Posterior subcapsular lens changes
Vitreous may be syneretic and contain clusters of cloudy fibrils Confluent
arcuate equatorial areas of chorioretinal degeneration
During late childhood, sharply demarcated, circular areas of chorioretinal degeneration in the midperiphery can be detected. There may be increased pigmentation around the margins of these lesions. During the second decade, the lesions enlarge, coalesce, and extend toward the posterior pole of the retina (Fig. 8). By the third decade, much of the retina is involved, although foveal lesions are rarely present until very late in the course of the disease. Histologic examination of an affected retina has shown focal areas of photoreceptor atrophy with adjacent retinal pigment epithelial hyperplasia. Electron microscopy has revealed mitochondrial abnormalities of the photoreceptors.114
|
The electroretinogram (ERG) eventually diminishes in amplitude and is usually extinguished well before the entire retina is involved clinically.91 The electro-oculogram becomes severely diminished, parallel to the reduction in the ERG.91
The slow progression of the degenerative changes in gyrate atrophy and the difficulty in measuring small changes in ocular function objectively make evaluation of any therapy difficult.110,115–119 Two therapeutic approaches have been attempted in patients with gyrate atrophy: reducing the accumulation of ornithine and stimulation of residual ornithine aminotransferase activity. Arginine is the precursor of ornithine. A chronic diet restricted in arginine has been shown to lower ornithine levels and slow the progression of the ocular disease.120 Some patients respond to pharmacologic doses of pyridoxine (vitamin B6) to increase the level of pyridoxal phosphate, a cofactor of OATase. A different single nonsense mutation within the OATase gene leads to a difference in the affinity of the enzyme for pyridoxal phosphate, accounting for the positive response to pyridoxine in this subset of patients.121,122
OCULOCEREBRORENAL SYNDROME (LOWE SYNDROME)
Renal tubular dysfunction, mental retardation, and congenital ocular defects form the triad of a rare disorder originally described by Lowe and coworkers and known as the oculocerebrorenal syndrome.123 The condition is transmitted in a sex-linked recessive pattern.124 The aminoaciduria is renal in origin; the plasma amino acids are normal. The enzyme responsible for this syndrome is an inositol polyphosphate 5-phosphatase within the Golgi apparatus whose gene has been mapped to the X chromosome.125–127
Systemic manifestations of this disorder usually are present at birth; they consist of hypotonia, failure to thrive, anorexia, and vomiting.123 Later signs are mental retardation, intention tremors, and a peculiar high-pitched cry. The renal tubular defect results in aminoaciduria, glycosuria, proteinuria, acidosis, phosphaturia, and hypophosphatemia with or without rickets. Many affected males also display a characteristic maladaptive behavior pattern that includes tantrums and stubbornness; this is not simply reflective of the developmental impairment or multiple disabilities present in these patients.128,129
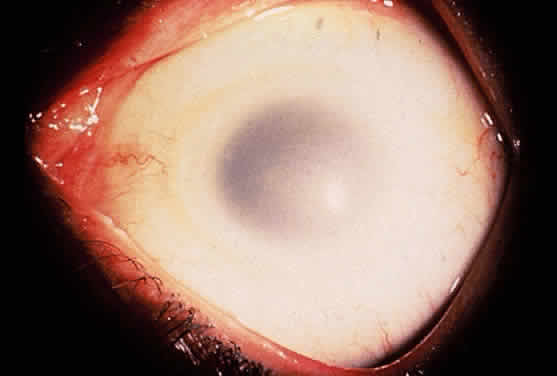
The ophthalmologic findings are among the earliest, most prominent and constant clinical features of this disorder.130,131 In a large review series, Abbassi and colleagues found that dense bilateral nuclear or posterior cortical cataracts were present at birth in nearly 100% (68/70) of the cases.124 Glaucoma was not as constant a finding but was present in 66% of cases.124
The cataract in Lowe syndrome is characterized by reduced anterior-posterior and equatorial measurements and malformations ranging from a discoid configuration to a giant posterior lentiglobus.130 Anteriorly, focal areas of lens capsule thickening and localized areas of lens epithelium hyperplasia are present. Punctate cortical opacities can be seen in the lens of female carriers of the disease and increase with age.132,133
Glaucoma, with or without buphthalmos, is usually ascribed to an anomalous development of the anterior chamber angle.131 Corneal opacities, present in at least 50% of cases, are probably related mainly to congenital and chronic glaucoma.131
There is no known effective treatment for the basic disorder; therapy consists of the correction of acidosis and rickets, if present.