| Amniocentesis was introduced in the 1930s as a diagnostic aid for placental
localization by amniography.19 It gained widespread acceptance as a technique in the prenatal diagnosis
of genetic diseases in the 1950s, after successful reports of amniotic
fluid analysis in cases of Rh isoimmunization.20–22 In 1956, Fuchs and Riis23 demonstrated the feasibility of fetal sex determination by examining X-chromatin
bodies in amniotic fluid cells. The ability to culture
amniotic fluid cells in tissue culture and to acquire sufficient viable
cells for karyotype analysis and biochemical studies was demonstrated
in 1966.24 A partial list of conditions that lend themselves to prenatal diagnosis
by amniocentesis is found in Table 3; all of these disorders have ocular manifestations.
TABLE 3. Disorders Diagnosed by Amniocentesis with Ocular Manifestations
| Chromosomal abnormalities |
| Down syndrome |
| Klinefelter's syndrome |
| Turner's syndrome |
| Neural tube defects |
| Anencephaly |
| Meningomyelocele |
| Metabolic diseases |
| Arginosuccinic aciduria |
| Cystinosis |
| Fabry's disease |
| Farber's disease |
| Fucosidosis |
| Galactosemia, type I |
| Galactokinase deficiency |
| Gaucher's disease |
| GM1 (generalized) gangliosidosis |
| Glucose-6-phosphate dehydrogenase deficiency |
| Glycogen storage disease, type I |
| GM2 gangliosidosis, type I (Tay-Sachs) |
| GM2 gangliosidosis, type II (Sandhoff's) |
| Hemoglobinopathies |
| Hemophilia |
| Hunter's syndrome |
| Hurler's syndrome |
| Hypervalinemia |
| Hypophosphatasia |
| Juvenile GM1 gangliosidosis |
| Krabbe's disease |
| Maple syrup urine disease |
| Maroteaux-Lamy syndrome |
| Metachromatic leukodystrophy (two forms) |
| Morquio's syndrome |
| Neimann-Pick disease (four types) |
| Porphyria |
| Refsum's disease |
| Sandhoff's disease |
| Sanfilippo's syndrome |
| Scheie's syndrome |
| Tay-Sachs disease |
| Thalassemia |
| Xeroderma pigmentosum |
From Spaeth GL, Nelson LB, Beadoin AR: Ocular teratology. In Duane TD, Jaeger
EA, eds. Biomedical Foundations of Ophthalmology. Philadelphia, JB
Lippincott, 1982.
Traditional genetic amniocentesis is usually offered between 15 and 20 weeks' gestation. Amniocentesis performed earlier has a higher complication
rate, as well as more amniotic culture failures. It may be
offered when prenatal maternal screening results are high risk for a genetic
abnormality or as an elective diagnostic test such as in advanced
maternal age or prior history of an aneuploidy (see Fig. 2). 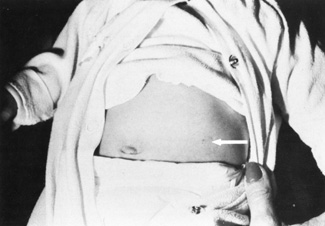 Fig. 2. Left midabdomen of neonate shows indented area probably resulting from
contact of the amniocentesis needle with the abdominal skin. Fig. 2. Left midabdomen of neonate shows indented area probably resulting from
contact of the amniocentesis needle with the abdominal skin.
|
Many large, multicenter studies have confirmed the safety of genetic amniocentesis, as
well as its cytogenetic diagnostic accuracy (greater
than 99%).2 The fetal loss rate is approximately 0.5%, and minor complications
occur infrequently. Table 4 lists known complications for amniocentesis.
TABLE 4. Complications and Their Incidence in Amniocentesis
| Fetal loss 0.5% |
| Vaginal spotting 1%–2 % |
| Amniotic fluid leakage 1%–2% |
| Chorioanmionitis 0.1% |
| Needle injury rare (Fig. 2) |
| Amniotic fluid cell culture failure rare | The procedure is performed under ultrasound guidance. After obtaining informed
consent, an ultrasound examinations is performed to establish
fetal viability, placental and fetal location, and depth to the largest
pocket of amniotic fluid (Fig. 3). The maternal abdomen is prepped aseptically and a local anesthetic
may be administered. A small gauge needle is then used to aspirate
approximately 10 to 20 mL of amniotic fluid. The availability of the
results is dependent on the amount of time needed for cell culture growth
but usually is available within 7 to 10 days. The results received
are a full cytogenic karyotype. 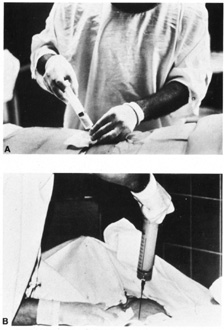 Fig. 3. A and B: Withdrawal of amniotic fluid with 20-gauge needle and 30-mL
syringe. Fig. 3. A and B: Withdrawal of amniotic fluid with 20-gauge needle and 30-mL
syringe.
|
Fluorescent in situ hybridization (FISH) is a new technology utilizing fluorescently
labeled DNA probes to detect or confirm gene or chromosome abnormalities.25,26 The sample DNA is first denatured, a process that separates the complementary
strands within the DNA double helix structure. The fluorescently
labeled probe of interest is then added to the denatured sample mixture
and hybridizes with the sample DNA at the target site as it reforms
itself back into a double helix. The probe signal can then be seen
through a fluorescent microscope and the sample DNA scored for the presence
or absence of the signal. FISH can be used in interphase cells to determine the chromosome number
or more chromosomes, as well as detect some specific chromosome rearrangements
that are characteristic for certain cancers. The primary advantage
of interphase FISH is that it can be performed rapidly if necessary, usually
within 24 hours, because cell growth is not required. A good example is the Aneuploid Screen Test that is performed on amniotic
fluid cells when there is a strong clinical indication for one of the
common trisomies. The sample nuclei are denatured and hybridized with
DNA probes for chromosomes 13, 18, 21, X, and Y. CHORIONIC VILLUS SAMPLING The indications for CVS are similar for amniocentesis, except for a few
rare genetic conditions that require chorionic villi for diagnosis.2 CVS is generally performed at 10 to 12 weeks' gestation. Similar
to other first trimester methods, CVS allows for results earlier that
can provide reassurance or allow for earlier and safer methods of pregnancy
termination. Similar to amniocentesis, CVS is performed under ultrasound
guidance. It can be performed either transabdominally or transcervically (Fig 4). Table 5 lists the contraindications and relative contraindications for CVS.
TABLE 5. Contraindications and Relative Contraindications for Chorionic
Villus Sampling
| Active cervical infection (Chlamydia or herpes)—contraindicated |
| Vaginal infection—relative contraindication |
| Vaginal bleeding or spotting—relative contraindication |
| Extreme anteversion or retroversion of the uterus—relative contraindication |
| Patient body habitus linking visualization—relative contraindication | 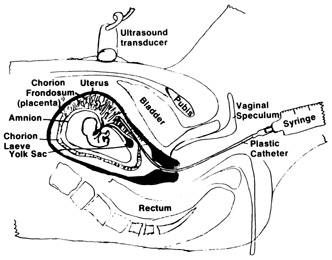 Fig. 4. Aspiration method of chorionic villi sampling. Ultrasound is used to localize
the chorionic frondosum and guide the aspirating catheter into
position. Syringe suction is used to trap and withdraw villi into the
catheter. Fig. 4. Aspiration method of chorionic villi sampling. Ultrasound is used to localize
the chorionic frondosum and guide the aspirating catheter into
position. Syringe suction is used to trap and withdraw villi into the
catheter.
|
Patients considering CVS should be counseled that there may be a slightly
higher risk of pregnancy loss associated with CVS than with traditional
amniocentesis. Pregnancy loss rates are reported to be 0.6% to 0.8% for
CVS in excess of traditional amniocentesis. Loss
may result from the procedure itself, but may incorporate the expected
spontaneous loss rate between 9 and 16 weeks of gestation. According
to the World Health Organization, the incidence of limb reduction defects
are approximately 6 per 10,000, which is not significantly different
from the incidence in the general population.2 Oromandibular-limb hypogenesis appeared to be more common with
CVS, although highest when CVS is performed before 9 weeks' gestation.27–29 Similar to amniocentesis, cytogenetics can be available in 7 to 10 days (Fig. 5). FISH can also be used to provide a limited aneuploid screen in 24 hours. 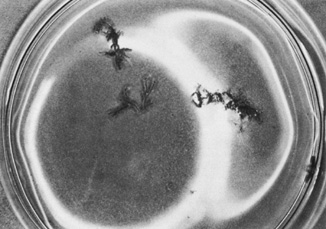 Fig. 5. Freshly aspirated chorionic villi in tissue culture medium in a Petri dish. Note
the branched structure. Fig. 5. Freshly aspirated chorionic villi in tissue culture medium in a Petri dish. Note
the branched structure.
|
CORDOCENTESIS Cordocentesis is also known as percutaneous umbilical blood sampling (PUBS). Under
direct ultrasound guidance, the umbilical vein is
punctured. This procedure cannot be performed before 18 weeks' gestation. A
karyotype of fetal blood can be available within 24 to 48 hours. Procedure-related pregnancy loss is less than 2%. Cordocentesis
is rarely used for cytogenetics. This procedure is utilized
more to evaluate fetal platelets, Rh sensitivity, and to administer
fetal medications.2 ULTRASOUND Diagnostic ultrasound is widely used in the assessment of pregnancy and
the fetus. Although clinical benefits of routine ultrasonography during
pregnancy have not been established, approximately 70% of pregnancies
in the United States undergo ultrasound evaluation.30 Because most instruments used in diagnostic ultrasonography produce energies
no greater than 10 to 20 mW cm2 (safety defined as less than 100 mW cm2), ultrasound is considered generally safe. No harmful biologic effects
on instrument operators, pregnant women, fetuses, or other patients
have been found. Infants exposed in utero have shown no significant differences in birth weight or length, childhood
growth, cognitive function, acoustic or visual ability, or rates
of neurologic deficits (see Fig. 6). 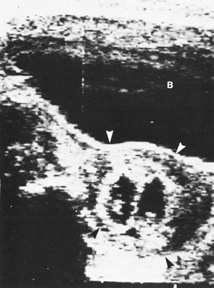 Fig. 6. A: First-trimester twin intrauterine gestations. Ultrasound examination
of the pregnant uterus (arrowheads) shows the “owl eyes” characteristic of early twin pregnancies. B: Maternal urinary bladder. (Courtesy of Alfred B. Kurtz, MD) Fig. 6. A: First-trimester twin intrauterine gestations. Ultrasound examination
of the pregnant uterus (arrowheads) shows the “owl eyes” characteristic of early twin pregnancies. B: Maternal urinary bladder. (Courtesy of Alfred B. Kurtz, MD)
|
Indications for ultrasound are listed in Table 6. There are several levels of ultrasound. A basic ultrasound suffices for
most obstetric patients. Table 7 lists the components of a basic ultrasound. A comprehensive ultrasound
may be indicated for a patient who is suspected of carrying a physiologically
or anatomically defective fetus by history, clinical evaluation, or
prior ultrasound examination. Because of the level of expertise
needed, comprehensive examinations are usually performed at a tertiary
center.
TABLE 6. Indications for Ultrasonography During Pregnancy
| Estimation of gestational age for patients with uncertain clinical dates, or
verification of dates for patients who are to undergo scheduled
elective repeat cesarean delivery, indicated induction of labor, or other
elective termination of pregnancy |
| Evaluation of fetal growth |
| Vaginal bleeding of undetermined etiology in pregnancy |
| Determination of fetal presentation |
| Suspected multiple gestation |
| Adjunct to amniocentesis |
| Significant uterine size/clinical dates discrepancy |
| Pelvic mass |
| Suspected hydatidiform mole |
| Adjunct to cervical cerclage placement |
| Suspected ectopic pregnancy |
| Adjunct to special procedures |
| Suspected fetal death |
| Suspected uterine abnormality |
| Intrauterine contraceptive device localization |
| Biophysical evaluation for fetal well-being |
| Observation of intrapartum events |
| Suspected polyhydramnios or oligohydramnios |
| Suspected abruption placentae |
| Adjunct to external version from breech to vertex presentation |
| Estimation of fetal weight and/or presentation in premature rupture
of membranes and/or premature labor |
| Abnormal serum alpha-fetoprotein value |
| Follow-up evaluation of placental location for identified placenta
previa |
| History of previous congenital anomaly |
| Serial evaluation of fetal growth in multiple gestation |
| Evaluation of fetal condition in late registrants for prenatal care |
Adapted from U.S. Department of Health and Human Services. Diagnostic ultrasound
in pregnancy. National Institutes of Health publication no. 84-667. Bethesda: National Institutes of Health, 1984.
TABLE 7. Components of Basic Ultrasound Examination
| Fetal number (Fig. 6) |
| Fetal presentation |
| Documentation of fetal life |
| Placental location |
| Assessment of amniotic fluid volume |
| Assessment of gestation age |
| Survey of fetal anatomy for gross malformations |
| Evaluation for maternal pelvic masses | FIRST TRIMESTER ULTRASOUND First trimester ultrasound may be performed transabdominally or transvaginally. Table 7 lists
the components of a first trimester ultrasound. A
crown–rump length, done between 7 and 13 weeks, can define a
gestational age to within 5 days (Fig. 7). 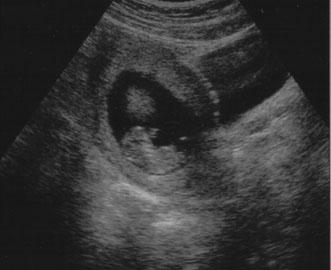 Fig. 7. First trimester ultrasound showing crown–rump length. Fig. 7. First trimester ultrasound showing crown–rump length.
|
Nuchal edema is an echo-free space between the skin line and the
soft tissue overlying the cervical spine. Nuchal edema is caused by subcutaneous
accumulation of fluid and has diverse etiology, including
aneuploidies, cardiovascular and pulmonary defects, skeletal dysplasias, congenital
infections, and hematologic and metabolic disorders. A nuchal
translucency (NT) is obtained between 10 and 13 weeks' 6 days' gestational age (Fig. 8).31 A study at King's College Hospital in London found an NT of 3 mm
was associated with a 4-times increase in the maternal age related
risk for aneuploidy. An NT greater than 4 mm resulted in a 29 times
increased risk for trisomies 21, 18, and 13. Additionally, with a 4 mm
or more NT, there was a high incidence of other anomalies and poor
prognosis, whereas with just 3 mm and a normal karyotype, the outcome
was usually normal. Table 8 lists the disorders associated with an increased nuchal translucency thickness.
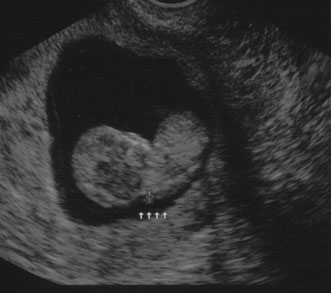 Fig. 8. Normal nuchal translucency of 0.19 cm at 11 weeks' gestation. Fig. 8. Normal nuchal translucency of 0.19 cm at 11 weeks' gestation.
|
TABLE 8. Disorders Associated with Increased Nuchal Translucency
| Down syndrome |
| Trisomy 18, 13 |
| Turner syndrome |
| Cardiac septal defect |
| Diaphragmatic hernia |
| Omphalacele |
| Arthrogryposis |
| Noonan's syndrome |
| Smith-Lemli-Opitz syndrome |
| Stickler syndrome |
| Jarco-Levine syndrome |
| Miller-Dieker syndrome |
| Amnion disruption sequence |
| Various skeletal dysplasias | The First Trimester Maternal Serum Biochemistry and Fetal Nuchal Translucency
Screening Study is looking at combining NT, maternal age, gestational
age, PAPP-A, and β-hCG to calculate a Down syndrome
and trisomy 18 risk by using computer software. Currently still
under investigation, this method is widely used in Europe. Women who
screen positive are then offered CVS or amniocentesis. SECOND TRIMESTER ULTRASOUND A second trimester ultrasound is usually done at 20 to 22 weeks' gestational
age. The most commonly used fetal measurements are biparietal
diameter, length of the femur or other long bones, and abdominal and
head circumference. In addition to measurements, an anatomic survey
is also done to evaluate the fetal brain (Fig. 9), spine, stomach, heart, kidneys, placental location and assessment
of amniotic fluid (Fig. 10). If maternal risk factors are present, tetra screening results are
abnormal, or there are abnormal findings on the anatomic survey, the
patient is sent for a comprehensive ultrasound. The components of a
comprehensive ultrasound are shown in Table 9. The ultrasound findings associated with Down syndrome include cardiac
defects or enlargement, cystic hygroma (Fig. 11), duodenal atresia (Fig. 12), omphalocele, polyhydramnios, choroids plexus cyst, and renal calyceal
dilation. 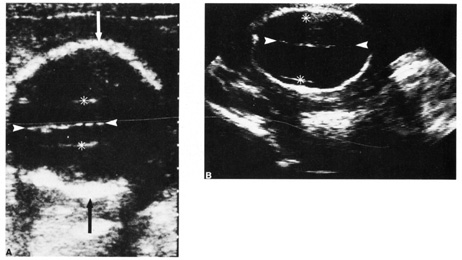 Fig. 9. Transaxial ultrasound of fetal heads. Fig. 9. Transaxial ultrasound of fetal heads.
|
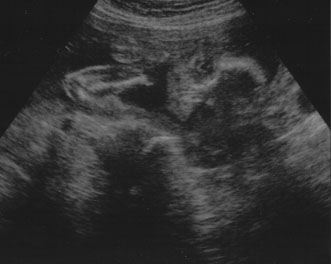 Fig. 10. Third trimester ultrasound image showing fetal ocular anatomy. Fig. 10. Third trimester ultrasound image showing fetal ocular anatomy.
|
TABLE 9. Components of a Comprehensive (Level II) Ultrasound
| Data | Placenta |
| Fetal number | Location |
| Presentation | Grade |
| Gender | Cord vessels and insertion |
| Cardiac activity | Fluid |
| | |
| Anatomy | Doppler |
| Cranial signs of neural tube defect | Umbilical artery |
| Choroid plexus | Left uterine artery |
| Ventricles | Right uterine artery |
| Four-chamber heart | Middle cerebral artery |
| Left ventricular outflow tract | |
| Right ventricular outflow tract | Measurements |
| Diaphragm | Biparietal diameter |
| Stomach | Head circumference |
| Bladder | AL |
| Kidneys | Foot length |
| Spine | Outer orbital diameter |
| Bowel echogenecity | Cerebellar diameter |
| Extremeties | Humerus |
| Abdominal wall | |
| Midface | Weight |
| Facial profile | |
| Lenses | Uterine/adnexal pathology |
| Fifth digit | |
| | Biophysical profile | 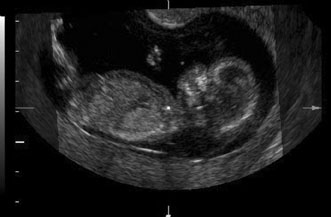 Fig. 11. Early second trimester ultrasound showing posterior neck cystic mass consistent
with cystic hygroma. Image courtesy of GE Medical Systems. Fig. 11. Early second trimester ultrasound showing posterior neck cystic mass consistent
with cystic hygroma. Image courtesy of GE Medical Systems.
|
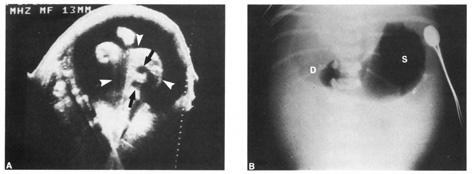 Fig. 12. Duodenal atresia in a second trimester fetus. A: Ultrasound scan of fetal abdomen (arrowheads) showing two fluid-filled structures (arrows). Increased amniotic fluid (polyhydramnios) surrounds the
fetus. B: Newborn radiograph of upper abdomen demonstrating gas-filled stomach (S) and duodenum (D), which are typical findings of duodenal atresia. (Courtesy
of Alfred B. Kurtz, MD) Fig. 12. Duodenal atresia in a second trimester fetus. A: Ultrasound scan of fetal abdomen (arrowheads) showing two fluid-filled structures (arrows). Increased amniotic fluid (polyhydramnios) surrounds the
fetus. B: Newborn radiograph of upper abdomen demonstrating gas-filled stomach (S) and duodenum (D), which are typical findings of duodenal atresia. (Courtesy
of Alfred B. Kurtz, MD)
|
THREE-DIMENSIONAL ULTRASOUND Three-dimensional ultrasound is currently investigational. It is
most commonly used at tertiary care centers and is commercially available
for patients to obtain a keepsake image of their unborn child. Potential
advantages include the ability to visualize fetal anatomy better
and possibly change a patient's diagnosis through improved visibility (Figs. 13 and 14). No confirmed adverse biologic effects on patients or instrument
operators caused by exposure have been demonstrated.32 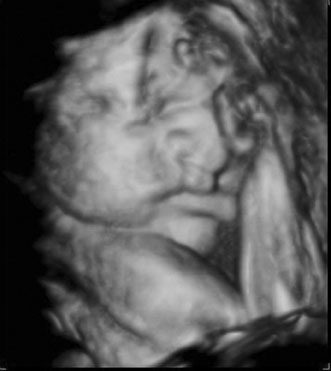 Fig. 13. Three-dimensional ultrasound image showing midline facial cleft. Image
courtesy of GE Medical Systems. Fig. 13. Three-dimensional ultrasound image showing midline facial cleft. Image
courtesy of GE Medical Systems.
|
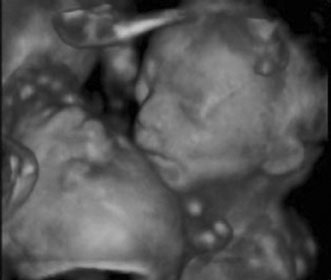 Fig. 14. Three-dimensional ultrasound image of twin gestation. Image courtesy
of GE Medical Systems. Fig. 14. Three-dimensional ultrasound image of twin gestation. Image courtesy
of GE Medical Systems.
|
MAGNETIC RESONANCE IMAGING Magnetic resonance imaging (MRI) is currently under investigation
for use in prenatal diagnosis. Advantages of MRI over ultrasound
include excellent tissue contrast, a large field of view, and relative
operator independence.33 Table 10 lists the indications of fetal MRI. One of the most successful areas has
been in the evaluation on the brain and central nervous system.
TABLE 10. Indications of Fetal Magnetic Resonance Imaging
| Central nervous system | Tumors |
| Hydrocephalus | Cervical teratomas |
| Mild, borderline ventricular dilation | Sacrococcygeal teratomas |
| Posterior fossa abnormalities | Intracranial tumors |
| Migration abnormalities | Other tumors |
| Suspected ischemia | |
| Vascular accidents, thrombosis | Placental abnormalities |
| Tumors | Invasive placenta (accrete, increta, percreta) |
| Spinal abnormalities | Chorioangioma |
| Tumors | Molar pregnancy |
| Spinal abnormalities | |
| Follow-up of prenatal surgery | Twins |
| | Twin transfusion syndrome |
| Thoracic abnormalities | Conjoined twins |
| Diaphragmatic hernia | |
| Cystic adenomatoid malformation | Maternal conditions |
| Sequestration | Unusual fibroids |
| | dnexal masses |
| Extremity, posturing abnormalities | Liver, CNS abnormalities, HELLP syndrome |
| Arthrogryposis | |
| Limb body wall | Evaluation of fetal well-being |
| | Fetal weight assessment |
| Abdominal abnormalities | Fetal CNS ischemia |
| Bowel obstruction | |
| Liver abnormalities, tumors | Other conditions |
| Abdominal wall defects | Abdominal pregnancy |
| | Tumors of any origin |
| | As replacement for early postnatal MRI |
CNS, central nervous system; HELLP syndrome, hemolysis, elevated liver
enzymes, and low platelet count syndrome; MRI, magnetic resonance imaging.
GENETIC COUNSELING/PRECONCEPTION COUNSELING Genetic counselors serve as the link between the medical communities' increasing
knowledge of genetics and a patient's understanding
of genetic risk. A genetic counselor helps a health care provider and
a patient understand the risks associated with birth defects and hereditary
disorders through interpreting family history, laboratory results, and
other medical information. Often times, a couple proceeds no
further with diagnostic testing after receiving a risk assessment. Ideally, the
couple planning pregnancy meets with a health care provider
prior to pregnancy in order to assess risk. Decisions at this time are as difficult as those after diagnosis of a live-born
child with a genetic handicap, and similar psychologic
reactions can occur.34 After abortion of an affected fetus, both parents—especially the
mother—may also require supportive psychological counseling.35 Properly applied, prenatal diagnosis with attendant genetic counseling
can be a powerful preventive medical tool. In the more personal sense, it
frequently allows at-risk couples to have healthy children
when they might otherwise forfeit the opportunity. |