|
|
Chapter 11: Glaucoma
Authors:
Glaucoma
Glaucoma is characterized by elevated intraocular pressure associated with optic disk cupping and visual field loss. In the majority of cases, there is no associated ocular disease (primary glaucoma) (Table 11-1).
Approximately 20,000 Americans are blind from glaucoma, making it the leading cause of preventable blindness in the United States. An estimated 2 million Americans have glaucoma. Primary open-angle glaucoma, the most common form, causes insidious asymptomatic progressive bilateral visual loss that is often not detected until extensive field loss has already occurred. Other forms of glaucoma are responsible for severe visual morbidity in individuals of all ages. Acute (angle-closure) glaucoma comprises 10-15% of cases in Caucasians. This percentage is much higher in Asians, particularly among the Burmese and Vietnamese in Southeast Asia.
The mechanism of raised intraocular pressure in glaucoma is impaired outflow of aqueous resulting from abnormalities within the drainage system of the anterior chamber angle (open-angle glaucoma) or impaired access of aqueous to the drainage system (closed-angle glaucoma) (Table 11-2). Treatment is directed toward reducing the intraocular pressure and, when possible, correcting the underlying cause.
Reducing aqueous production is a method of reducing intraocular pressure used in all forms of glaucoma. Several medications reduce aqueous production. Surgical procedures that reduce aqueous production are available but are generally used only after medical treatment has failed. Facilitating flow of aqueous through the trabecular meshwork is useful in open-angle glaucoma. Improving access of aqueous to the anterior chamber angle in closed-angle glaucoma may be achieved by peripheral laser iridotomy or surgical iridectomy if the cause is pupillary block, miosis if there is angle crowding, or cycloplegia if there is anterior lens displacement. Surgically bypassing the drainage system is useful in open-angle glaucoma and in angle closure that fails to respond to medical treatment. In the secondary glaucomas, consideration must always be given to treating the primary abnormality.
In all patients with glaucoma, the necessity for treatment and its effectiveness are assessed by regular determination of intraocular pressure (tonometry), inspection of optic disks, and measurement of visual fields.
The management of glaucoma is best left to the ophthalmologist, but the magnitude of the problem and the importance of detecting asymptomatic cases call for the cooperation and assistance of all medical personnel. Ophthalmoscopy (noting optic nerve changes) and tonometry should be part of the routine ophthalmologic examination of all patients old en-ough to cooperate and certainly all patients over 30 years of age. This is especially important in patients with a family history of glaucoma.
PHYSIOLOGY OF AQUEOUS HUMOR
The intraocular pressure is determined by the rate of aqueous production and the resistance to outflow of aqueous from the eye. Some knowledge of the physiology of aqueous humor is necessary for understanding glaucoma.
Composition of Aqueous
The aqueous is a clear liquid that fills the anterior and posterior chambers of the eye. Its volume is about 250 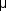 L, and its rate of production, which is subject to diurnal variation, is about 2.5
L, and its rate of production, which is subject to diurnal variation, is about 2.5 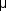 L/min. The osmotic pressure is slightly higher than that of plasma. The composition of aqueous is similar to that of plasma except for much higher concentrations of ascorbate, pyruvate, and lactate and lower concentrations of protein, urea, and glucose.
L/min. The osmotic pressure is slightly higher than that of plasma. The composition of aqueous is similar to that of plasma except for much higher concentrations of ascorbate, pyruvate, and lactate and lower concentrations of protein, urea, and glucose.
Formation & Flow of Aqueous
Aqueous is produced by the ciliary body. An ultrafiltrate of plasma produced in the stroma of the ciliary processes is modified by the barrier function and secretory processes of the ciliary epithelium. Entering the posterior chamber, the aqueous passes through the pupil into the anterior chamber (Figure 11-1) and then to the trabecular meshwork in the anterior chamber angle. During this period, there is some differential exchange of components with the blood in the iris.
Intraocular inflammation or trauma causes an increase in the protein concentration. This is called plasmoid aqueous and closely resembles blood serum.
Outflow of Aqueous
The trabecular meshwork is composed of beams of collagen and elastic tissue covered by trabecular cells that form a filter with a decreasing pore size as the canal of Schlemm is approached. Contraction of the ciliary muscle through its insertion into the trabecular meshwork increases pore size in the meshwork and hence the rate of aqueous drainage. Passage of aqueous into Schlemm's canal depends upon cyclic formation of transcellular channels in the endothelial lining. Efferent channels from Schlemm's canal (about 30 collector channels and 12 aqueous veins) conduct the fluid into the venous system. A small amount of aqueous passes between the bundles of the ciliary muscle and through the sclera (uveoscleral flow) (Figure 11-1).
The major resistance to aqueous outflow from the anterior chamber is the endothelial lining of Schlemm's canal and the adjacent portions of the trabecular meshwork-rather than the venous collector system. But the pressure in the episcleral venous network determines the minimum level of intraocular pressure that can be achieved by medical therapy.
Pressure Dynamics
Intraocular pressure is such an important feature of glaucoma that a review of pressure-tension-strain relationships is desirable for elucidation of the possible mechanisms of neuronal damage.
A. Pressure:
Hydrostatic pressure is the force per unit area exerted by a fluid (gas or liquid) within a closed space. With the eye, as with other fluid-filled closed systems, the pressure force is exerted normal to the structural wall (the corneoscleral wall). Average pressure in the eye is about 14 mm Hg. For calculations, centimeters of water is a more convenient unit of pressure than millimeters of mercury. To convert millimeters of mercury to centimeters of water, multiply by 1.36. In more familiar terms, the average eye pressure is about 19 cm (7.5 inches) of water, or 0.25 psi (pounds per square inch). Glaucomatous damage usually begins at roughly double that value, and the eye ruptures at about 240 times average values.
Hydrostatic pressure per se causes no damage to the delicate neurons paralleling the scleral wall. A diver lying on the ocean bottom may be compared to a neuron lying on the uveoscleral bed. The diver will perceive no discomfort at a depth of 43 meters (141 feet) even though the pressure is about 3000 mm Hg, or approximately the pressure within an eye that results in rupture. The diver's body-though subjected to about 10 tons of hydrostatic pressure-will not be pushed against the ocean floor, and a neuron is not pushed against the sclera by hydrostatic pressure.
B. Tension (Tensile Stress):
A jack supporting a car is subjected to compressive stress. A towline pulling a car is subjected to tensile stress, or tension. Stresses are assigned a magnitude of force per unit area. Tensile stress, or the tension force vector, acts parallel to the scleral wall (attempting to pull the sclera apart). In the same way, the pressure of the abdomen at right angles to a belt is almost analogous to intraocular pressure, while the tension along the belt acting to pull the belt apart is analogous to scleral tension.
Trampolines and drumheads are examples of pure tension without pressure. The pressure is the same on either side of the tensed membrane. Tension levels in the sclera, cornea, and lamina cribrosa are not equal. The tension equation for thin-walled spheres can be used to obtain a close approximation of tensions in various parts of the corneoscleral wall. Tension in the sclera is directly proportionate to the intraocular pressure multiplied by the radius of curvature of the sclera and inversely proportionate to twice the thickness of the sclera:

An inflated surgical glove or balloon (Figure 11-2) illustrates this relationship. The palm of the glove has relatively high tension and the thumb relatively low tension, though the pressure within the glove is equal at all locations. The thumb has low tension because the radius of curvature is small and the thickness large relative to the same factors at the palm. In the eye, tension is lower in the cornea or optic cup than in the sclera.
An eye under slowly increasing pressure usually ruptures beneath the lateral rectus where the sclera is thinner, as the tension equation would suggest. A precipitous pressure rise due to trauma (eg, a blow from a club) frequently ruptures the eye at the limbus owing to the anvil effect of the more viscous vitreous.
C. Strain:
Strain is stretch or displacement per unit length. A strain gauge measures displacement. Strain can result in damage and in the body can cause both pain and damage. Using the belt analogy, strain is the stretch per unit length of the belt resulting from the tension in the belt caused by the pressure of the abdomen.
Young's modulus E is used for determining the elastic properties of structures such as cables, pressure vessels, submarines, biologic cells, unicellular organisms, and eyes. E is defined as the tension required to stretch a material of unit cross section to double its original length. This is represented by the following equation:

Thus, the stretch of the sclera per unit length (strain) is derived by dividing the change in tension of the sclera by Young's modulus of the sclera E.
The belt analogy may now be used to illustrate the way in which neurons are damaged in glaucoma. Envision a very obese person wearing a large belt (sclera) with a delicate cloth liner (neurons). After fasting for several days, the obese subject feasts heavily, with the result that there is some ripping of the delicate cloth liner. The progression of the damage process is as follows: (1) The expanding abdomen (intraocular pressure) exerts gentle pressure at right angles to the belt, producing a summation tension parallel to the belt (sclera), tending to pull the belt apart. (2) The tension leads to stretching (strain) of the belt, following the rules of Young's modulus. (3) The stretching (strain) results in damage to the delicate cloth liner (neurons).
PATHOPHYSIOLOGY OF GLAUCOMA
The pathophysiology of intraocular pressure elevation-whether due to open-angle or to angle-closure mechanisms-will be discussed as each disease entity is considered (see below). The effects of raised intraocular pressure within the eye are common to all forms of glaucoma, their manifestations being influenced by the time course and magnitude of the rise in intraocular pressure.
The major mechanism of visual loss in glaucoma is ganglion cell atrophy, leading to thinning of the inner nuclear and nerve fiber layers of the retina and axonal loss in the optic nerve. The optic disk becomes atrophic, with enlargement of the optic cup (see below). The iris and ciliary body also become atrophic, and the ciliary processes show hyaline degeneration.
In acute angle-closure glaucoma, the intraocular pressure reaches 60-80 mm Hg, resulting in ischemic damage to the iris with associated corneal edema and optic nerve damage.
CLINICAL ASSESSMENT IN GLAUCOMA
Tonometry
Tonometry is measurement of intraocular pressure. The most widely used instrument is the Goldmann applanation tonometer, which is attached to the slitlamp and measures the force required to flatten a fixed area of the cornea. Other applanation tonometers are the Perkins tonometer and the Tono-Pen, both of which are portable; and the pneumatotonometer, which is useful when the cornea has an irregular surface and can be used with a soft contact lens in place. The Schiotz tonometer is portable and measures the corneal indentation produced by a known weight. (For further discussion of tonometry, see Chapter 2; for tonometer disinfection techniques, see Chapter 21).
The normal range of intraocular pressure is 10-24 mm Hg. A single normal reading does not rule out glaucoma. In primary open-angle glaucoma, many affected individuals will have a normal intraocular pressure when first measured. Conversely, isolated raised intraocular pressure does not necessarily mean that the patient has primary open-angle glaucoma, since other evidence in the form of a glaucomatous optic disk or visual field changes is necessary for diagnosis. If the intraocular pressure is consistently elevated in the presence of normal optic disks and visual fields (ocular hypertension), the patient may be observed periodically as a glaucoma suspect.
Gonioscopy (See also Chapter 2)
The anterior chamber angle is formed by the junction of the peripheral cornea and the iris, between which lies the trabecular meshwork (Figure 11-3). The configuration of this angle-ie, whether it is wide (open), narrow, or closed-has an important bearing on the outflow of aqueous. The anterior chamber angle width can be estimated by oblique illumination with a penlight (Figure 11-4) or by slitlamp observation of the depth of the peripheral anterior chamber, but it is best determined by gonioscopy, which allows direct visualization of the angle structures (Figure 11-3). If it is possible to visualize the full extent of the trabecular meshwork, the scleral spur, and the iris processes, the angle is open. Being able to see only Schwalbe's line or a small portion of the trabecular meshwork means that the angle is narrow. Being unable to see Schwalbe's line means that the angle is closed.
Large myopic eyes have wide angles, and small hyperopic eyes have narrow angles. Enlargement of the lens with age narrows the angle and accounts for some cases of angle-closure glaucoma.
Race is also a factor. The angles of Southeast Asians are much narrower than those of Caucasians.
Optic Disk Assessment
The normal optic disk has a central depression-the physiologic cup-whose size depends on the bulk of the fibers that form the optic nerve relative to the size of the scleral opening through which they must pass. In hyperopic eyes, the scleral opening is small, and thus the optic cup is small; the reverse is true in myopic eyes. Glaucomatous optic atrophy produces specific disk changes characterized chiefly by loss of disk substance-detectable as enlargement of the optic disk cup-associated with disk pallor in the area of cupping. Other forms of optic atrophy cause widespread pallor without increased disk cupping.
In glaucoma, there may be concentric enlargement of the optic cup or preferential superior and inferior cupping with focal notching of the rim of the optic disk. The optic cup also increases in depth as the lamina cribrosa is displaced backward. As cupping develops, the retinal vessels on the disk are displaced nasally (Figure 11-5). The end result of glaucomatous cupping is the so-called "bean pot" cup in which no neural rim tissue is apparent (Figures 11-6 and 11-7).
The "cup-disk ratio" is a useful way of recording the size of the optic disk in glaucoma patients. It is the ratio of cup size to disk diameter, eg, a small cup is 0.1 and a large cup 0.9. In the presence of elevated intraocular pressure, a cup-disk ratio greater than 0.5 or significant asymmetry between the two eyes is highly suggestive of glaucomatous atrophy.
Clinical assessment of the optic disk can be performed by direct ophthalmoscopy or by examination with the 70-diopter lens, the Hruby lens, or special corneal contact lenses that give a three-dimensional view.
Other clinical evidence of neuronal damage in glaucoma is atrophy of the nerve fiber layer. This is detectable (Hoyt's sign) by ophthalmoscopy-particularly when red-free light is used-and precedes the development of optic disk changes.
Visual Field Examination
Regular visual field examination is essential to the diagnosis and follow-up of glaucoma. Glaucomatous field loss is not in itself specific, since it consists of nerve fiber bundle defects that may be seen in other forms of optic nerve disease; but the pattern of field loss, the nature of its progression, and the correlation with changes in the optic disk are characteristic of the disease.
Glaucomatous field loss involves mainly the central 30 degrees of field (Figure 11-8). The earliest change is baring of the blind spot. Contiguous extension into Bjerrum's area of the visual field-at 15 degrees from fixation-produces a Bjerrum scotoma and then an arcuate scotoma. Focal areas of more pronounced loss within Bjerrum's area are known as Seidel scotomas. Double arcuate scotomas-above and below the horizontal meridian-are often accompanied by a nasal step (of Roenne) because of differences in size of the two arcuate defects. Peripheral field loss tends to start in the nasal periphery as a constriction of the isopters. Subsequently, there may be connection to an arcuate defect, producing peripheral breakthrough. The temporal peripheral field and the central 5-10 degrees are affected late in the disease. Central visual acuity is not a reliable index of progress of the disease. In end-stage disease, there may be normal central acuity but only 5 degrees of visual field in each eye. In advanced glaucoma, the patient may have 20/20 visual acuity and be legally blind.
Various ways of testing the visual fields in glaucoma include the automated perimeter, the Goldmann perimeter, the Friedman field analyzer, and the tangent screen. (For technique and other details, see Chapter 2)
PRIMARY GLAUCOMA
PRIMARY OPEN-ANGLE GLAUCOMA
Primary open-angle glaucoma is the most common form. About 0.4-0.7% of persons over age 40 and 2-3% of persons over age 70 are estimated to have primary open-angle glaucoma. The disease is four times more common and generally more aggressive in blacks. There is a strong familial tendency in primary open-angle glaucoma, and close relatives of affected individuals should undergo regular screening.
The chief pathologic feature of primary open-angle glaucoma is a degenerative process in the trabecular meshwork, including deposition of extracellular mate-rial within the meshwork and beneath the endothelial lining of Schlemm's canal. This differs from the normal aging process. The consequence is a reduction in aqueous drainage leading to a rise in intraocular pressure.
Juvenile-onset open-angle glaucoma (a familial primary open-angle glaucoma with early onset), about 5% of familial cases of primary open-angle glaucoma, and about 3% of nonfamilial cases of primary open-angle glaucoma are associated with mutations in a gene on chromosome 1. This gene causes trabecular meshwork cells to produce an extracellular protein known as trabecular meshwork-inducible glucocorticoid response (TIGR) as a result of its association with glaucoma secondary to steroid therapy. It is suggested that these mutations result in abnormal amounts or types of TIGR. A mutation on chromosome 3 has also been implicated in autosomal dominant adult-onset primary open-angle glaucoma.
Raised intraocular pressure precedes optic disk and visual field changes by months to years. Although there is a clear association between the level of intraocular pressure and the severity and rate of progression of visual loss, there is great variability between individuals in the effect on the optic nerve of a given pressure elevation. Some people tolerate elevated intraocular pressure without developing disk or field changes (ocular hypertension; see below); others develop glaucomatous changes with consistently "normal" intraocular pressure (low-pressure glaucoma; see below).
The mechanism of neuronal damage in primary open-angle glaucoma and its relationship to the level of intraocular pressure is much debated. The major theories implicate intraocular pressure-dependent changes (as discussed above) or reduction in the vascular supply to the optic nerve head.
Higher levels of intraocular pressure are associated with greater field loss at presentation. When there is glaucomatous field loss on first examination, the risk of further progression is much greater. Since intraocular pressure is the only treatable risk factor, it remains the focus of therapy. There is strong evidence that control of intraocular pressure slows disk damage and field loss.
In the patient with extensive disk changes or field loss, it is advisable to reduce the intraocular pressure as much as possible, whereas a patient with only a suspicion of disk or field changes may need less vigorous treatment. In all cases, the inconveniences and possible complications of treatment must be considered. Many glaucoma patients are old and frail and may not tolerate vigorous treatment. In order to gain a perspective on the need for treatment, an initial period of observation without treatment may be necessary to determine the rate of progression of disk and field changes. There is no justification for subjecting an elderly patient to extremes of treatment when the likelihood of their developing significant visual loss during their lifetime is small.
Diagnosis
The diagnosis of primary open-angle glaucoma is established when glaucomatous optic disk or field changes are associated with elevated intraocular pressures, a normal-appearing open anterior chamber angle, and no other reason for intraocular pressure elevation. Approximately 50% of patients with primary open-angle glaucoma have a normal intraocular pressure when first examined, so repeated tonometry is necessary before the diagnosis can be established.
Screening for Glaucoma
The major problem in detection of primary open-angle glaucoma is the absence of symptoms until relatively late in the disease. When patients first notice field loss, substantial glaucomatous cupping has already occurred. If treatment is to be successful, it must be started early in the disease, and this depends upon an active screening program. Unfortunately, glaucoma screening programs are hampered by the unreliability of a single intraocular pressure measurement in the detection of primary open-angle glaucoma and the complexities of relying on optic disk or visual field changes. At present it is necessary to rely for early diagnosis on regular ophthalmologic assessment of first-degree relatives of affected individuals.
Medical Treatment of Glaucoma
A. Suppression of Aqueous Production:
Topical beta-adrenergic blocking agents are now the most widely used form of glaucoma therapy. They may be used alone or in combination with other drugs. Timolol maleate 0.25% and 0.5%, betaxolol 0.25% and 0.5%, levobunolol 0.25% and 0.5%, metipranolol 0.3%, and carteolol 1% are the currently available preparations. The major contraindications to their use are chronic obstructive airways disease-particularly asthma-and cardiac conduction defects. Betaxolol, with its relatively greater selectivity for 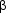 1 receptors, less often produces respiratory side effects but is also less effective at reducing intraocular pressure. Depression, confusion, and fatigue may occur with the topical beta-blocking agents.
1 receptors, less often produces respiratory side effects but is also less effective at reducing intraocular pressure. Depression, confusion, and fatigue may occur with the topical beta-blocking agents.
Apraclonidine is an  2-adrenergic agonist that decreases aqueous humor formation without effect on outflow. Epinephrine and dipivefrin have some effect on aqueous production (see below).
2-adrenergic agonist that decreases aqueous humor formation without effect on outflow. Epinephrine and dipivefrin have some effect on aqueous production (see below).
Brimonidine 0.2% is a new alpha-adrenergic agonist that primarily inhibits aqueous production and secondarily increases aqueous outflow. It shows promise both as a first-line antiglaucoma drug and as an adjunctive agent.
Dorzolamide hydrochloride 2% is a recently developed topical carbonic anhydrase inhibitor that is especially effective when employed adjunctively, though not as effective as systemic carbonic anhydrase inhibitors. Combining dorzolamide and timolol in the same solution is under investigation.
Systemic carbonic anhydrase inhibitors-acetazolamide is the most widely used, but dichlorphenamide and methazolamide are alternatives-are used in chronic glaucoma when topical therapy is insufficient and in acute glaucoma when very high intraocular pressure needs to be controlled quickly. They are capable of suppressing aqueous production by 40-60%. Acetazolamide can be administered orally in a dosage of 125-250 mg up to four times daily or as Diamox Sequels 500 mg once or twice daily, or it can be given intravenously (500 mg). The carbonic anhydrase inhibitors are associated with major systemic side effects that limit their usefulness for long-term therapy.
Hyperosmotic agents influence aqueous production as well as dehydrating the vitreous body (see below).
B. Facilitation of Aqueous Outflow:
Parasympathomimetic agents increase aqueous outflow by action on the trabecular meshwork through contraction of the ciliary muscle. Pilocarpine is the most commonly used drug in this group. It is given as 0.5-6% solution instilled several times a day or as 4% gel instilled at bedtime. Carbachol 0.75-3% is an alternative cholinergic agent. Irreversible anticholinesterase agents are the longest-acting parasympathomimetics available. These include demecarium bromide, 0.125% and 0.25%, and echothiophate iodide, 0.03-0.25%, which are generally restricted to aphakic or pseudophakic patients because of their cataractogenic potential. Caution: The irreversible anticholinesterase agents will potentiate succinylcholine administered during anesthesia, and anesthetists must be appropriately warned prior to surgery.
All parasympathomimetic agents produce miosis with dimness of vision, particularly in patients with cataract, and accommodative spasm that may be disabling to younger patients. Retinal detachment is a serious but rare occurrence.
Latanoprost 0.005%, a prostaglandin F2 analog, acts as an antiglaucoma drug by increasing the uveoscleral outflow of aqueous. It is used once daily in the evening and may work alone or as an adjunct to other antiglaucoma agents. To date there have been no systemic side effects, and once-daily dosing is a distinct advantage. However, it does increase iris pigmentation.
analog, acts as an antiglaucoma drug by increasing the uveoscleral outflow of aqueous. It is used once daily in the evening and may work alone or as an adjunct to other antiglaucoma agents. To date there have been no systemic side effects, and once-daily dosing is a distinct advantage. However, it does increase iris pigmentation.
Epinephrine, 0.25-2% instilled once or twice daily, increases aqueous outflow with some decrease in aqueous production. There are a number of external ocular side effects, including reflex conjunctival vasodilation, adrenochrome deposits, follicular conjunctivitis, and allergic reactions. Dipivefrin is a prodrug of epinephrine that is metabolized intraocularly to its active state. Neither epinephrine nor dipivefrin should be used in eyes with narrow anterior chamber angles.
C. Reduction of Vitreous Volume:
Hyperosmotic agents render the blood hypertonic, thus drawing water out of the vitreous and causing it to shrink. This is in addition to decreasing aqueous production. Reduction in vitreous volume is helpful in the treatment of acute angle-closure glaucoma and in malignant glaucoma when anterior displacement of the crystalline lens (caused by volume changes in the vitreous or choroid) produces angle closure (secondary angle-closure glaucoma).
Oral glycerin (glycerol), 1 mL/kg of body weight in a cold 50% solution mixed with lemon juice, is the most commonly used agent, but it should be used with care in diabetics. Alternatives are oral isosorbide and intravenous urea or mannitol (see Chapter 3 for dosages).
D. Miotics, Mydriatics, and Cycloplegics:
Constriction of the pupil is fundamental to the management of primary angle-closure glaucoma and the angle crowding of plateau iris. Pupillary dilation is important in the treatment of angle closure secondary to iris bombé due to posterior synechiae.
When angle closure is secondary to anterior lens displacement, cycloplegics (cyclopentolate and atropine) are used to relax the ciliary muscle and thus tighten the zonular apparatus in an attempt to draw the lens backward.
Surgical & Laser Treatment of Glaucoma
A. Peripheral Iridotomy and Iridectomy:
Pupillary block in angle-closure glaucoma is most satisfactorily overcome by forming a direct communication between the anterior and posterior chambers that removes the pressure difference between them. This is best done with the neodymium: YAG laser. Surgical peripheral iridectomy is performed if laser iridotomy is ineffective. YAG laser iridotomy is preventive when used in patients with narrow angles before closure attacks occur
B. Laser Trabeculoplasty:
Application of laser (usually argon) burns via a goniolens to the trabecular meshwork facilitates aqueous outflow by virtue of its effects on the trabecular meshwork and Schlemm's canal or cellular events that enhance the function of the meshwork. The technique is applicable to many forms of open-angle glaucoma, and the results are variable depending upon the underlying cause. The pressure reduction usually allows decrease of medical therapy and postponement of glaucoma surgery. Treatments can be repeated (see Chapter 24). Laser trabeculoplasty may be used in the initial treatment of primary open-angle glaucoma. In most cases, the intraocular pressure gradually returns to the pretreatment level 2-5 years later
C. Glaucoma Drainage Surgery:
The increased effectiveness of medical and laser treatment has reduced the need for glaucoma drainage surgery, but surgery is able to produce a more marked reduction in intraocular pressure.
Trabeculectomy is the procedure most commonly used to bypass the normal drainage channels, allowing direct access from the anterior chamber to the subconjunctival and orbital tissues. The major complication is fibrosis in the episcleral tissues, leading to closure of the new drainage pathway. This is most likely to occur in young patients, blacks, in patients with glaucoma secondary to uveitis, and in those who have previously undergone glaucoma drainage surgery or other surgery involving the episcleral tissues. Adjunctive treatment with antimetabolites such as fluorouracil and mitomycin reduces the risk of bleb failure but may lead to other bleb-related complications or maculopathy from persistent ocular hypotony.
Implantation of a silicone tube to form a permanent conduit for aqueous flow out of the eye is an alternative procedure for eyes that are unlikely to respond to trabeculectomy. This includes eyes with secondary glaucoma, particularly neovascular glaucoma, and glaucoma following corneal graft surgery.
Goniotomy is a useful technique in treating primary congenital glaucoma, in which there appears to be an obstruction to aqueous drainage in the internal portion of the trabecular meshwork.
D. Cyclodestructive Procedures:
Failure of medical and surgical treatment in advanced glaucoma may lead to consideration of laser or surgical destruction of the ciliary body to control intraocular pressure. Cryotherapy, diathermy, high-frequency ultrasound, and thermal mode neodymium:YAG laser therapy can all be used to cause destruction of the ciliary body.
Course & Prognosis
Without treatment, open-angle glaucoma may be insidiously progressive to complete blindness. If antiglaucoma drops control the intraocular pressure in an eye that has not suffered extensive glaucomatous damage, the prognosis is good (though visual field loss may progress in spite of normalized intraocular pressure). When the process is detected early, most glaucoma patients can be successfully managed medically.
NORMAL-PRESSURE GLAUCOMA (Low-Pressure Glaucoma)
A minority of patients with glaucomatous optic disk or visual field changes have an intraocular pressure consistently below 25 mm Hg. These patients have normal- or low-pressure glaucoma. The pathogenesis involves an abnormal sensitivity to intraocular pressure because of vascular or mechanical abnormalities at the optic nerve head. Disk hemorrhages are more frequently seen in normal-pressure than in primary open-angle glaucoma and often herald progression of field loss.
Before the diagnosis of low-pressure glaucoma can be established, a number of entities must be excluded:
Prior episode of raised intraocular pressure, such as caused by iridocyclitis, trauma, or topical steroid therapy.
Large diurnal variation in intraocular pressure with significant elevations, usually early in the morning.
Postural changes in intraocular pressure with a marked elevation when lying flat.
Intermittent elevations of intraocular pressure such as in subacute angle closure.
Other causes of optic disk and field changes, including congenital disk abnormalities and acquired optic atrophy due to tumors or vascular disease.
OCULAR HYPERTENSION
Ocular hypertension is elevated intraocular pressure without disk or field abnormalities and is more common than primary open-angle glaucoma. The rate at which such individuals develop glaucoma is approximately 5-10 per 1000 per year. The risk increases with increasing intraocular pressure, increasing age, a positive family history for glaucoma, myopia, diabetes mellitus, and cardiovascular disease. It is also increased in blacks. The development of disk hemorrhages in a patient with ocular hypertension also indicates an increased risk for development of glaucoma.
Patients with ocular hypertension are considered glaucoma suspects and should undergo regular monitoring (one to three times a year) of the optic disk, intraocular pressure, and visual fields.
PRIMARY ACUTE ANGLE-CLOSURE GLAUCOMA
Primary acute angle-closure glaucoma occurs when sufficient iris bombé develops to cause occlusion of the anterior chamber angle by the peripheral iris. This blocks aqueous outflow and the intraocular pressure rises rapidly, causing severe pain, redness, and blurring of vision. Angle-closure glaucoma occurs in eyes with preexisting anatomic narrowing of the anterior chamber angle (found mainly in hyperopes). The acute attack generally occurs in older patients when there has been enlargement of the crystalline lens associated with aging. In angle-closure glaucoma, the pupil is mid-dilated, with associated pupillary block. This usually occurs in the evenings, when the level of illumination is reduced. It may also occur with pupillary dilation for ophthalmoscopy. If pupillary dilation is necessary in a patient with a shallow anterior chamber (easily detected by oblique illumination with a penlight [Figure 11-4] and then confirmed by gonioscopy), it is best to rely on short-acting mydriatics and observe the patient carefully.
Clinical Findings
Acute angle-closure glaucoma is characterized by a sudden onset of severe blurring followed by excruciating pain, halos, and nausea and vomiting. Other findings include markedly increased intraocular pressure, a shallow anterior chamber, a steamy cornea, a fixed, moderately dilated pupil, and ciliary injection. It is important to perform gonioscopy on the fellow eye.
Differential Diagnosis
Acute iritis causes more photophobia than acute glaucoma. Intraocular pressure is usually not elevated; the pupil is constricted; and the cornea is usually not edematous. Marked flare and cells are present in the anterior chamber, and there is deep ciliary injection.
In acute conjunctivitis, there is little or no pain and no visual loss. There is discharge from the eye and an intensely inflamed conjunctiva but no ciliary injection. The pupillary responses and intraocular pressure are normal, and the cornea is clear.
Complications & Sequelae
If treatment is delayed, the peripheral iris may adhere to the trabecular meshwork (anterior synechiae), producing irreversible occlusion of the anterior chamber angle requiring surgery. Optic nerve damage is common.
Treatment
Acute angle-closure glaucoma is an ophthalmic emergency!
Treatment is initially directed at reducing the intraocular pressure. Intravenous and oral acetazol-amide-along with hyperosmotic agents and topical beta-blockers-will usually reduce the intraocular pressure. Pilocarpine 4% can then be used intensively, eg, 1 drop every 15 minutes for 1 hour. Epinephrine must not be used because it will accentuate angle closure.
Once the intraocular pressure is under control, peripheral iridectomy should be undertaken to form a permanent connection between the anterior and posterior chambers, thus preventing recurrence of iris bombé. This is most often done with the neo-dymium:YAG laser. Surgical peripheral iridectomy is indicated if laser treatment is unsuccessful.
In most cases, the fellow eye should undergo prophylactic laser iridotomy.
SUBACUTE ANGLE-CLOSURE GLAUCOMA
The same etiologic factors operate in subacute as in acute angle-closure glaucoma except that episodes of elevated intraocular pressure are of short duration and are recurrent. The episodes of angle closure resolve spontaneously, but there is accumulated damage to the anterior chamber angle, with formation of peripheral anterior synechiae. Subacute angle closure will occasionally progress to acute closure.
There are recurrent short episodes of unilateral pain, redness, and blurring of vision associated with halos around lights. Attacks often occur in the evenings and resolve overnight. Examination between attacks may show only a narrow anterior chamber angle. The diagnosis can be confirmed by gonioscopy.
Treatment is similar to that of primary angle- closure glaucoma.
CHRONIC ANGLE-CLOSURE GLAUCOMA
A small number of patients with the predisposition to a anterior chamber angle closure never develop episodes of acute rise in intraocular pressure but form increasingly extensive peripheral anterior synechiae accompanied by a gradual rise in intraocular pressure. These patients present in the same way as those with primary open-angle glaucoma, often with extensive visual field loss in both eyes. Occasionally, they have attacks of subacute angle closure.
On examination, there is elevated intraocular pressure, narrow anterior chamber angles with variable amounts of peripheral anterior synechiae, and optic disk and visual field changes.
Once again, peripheral iridectomy is an important component of treatment. (Laser iridotomy in these patients is liable to produce a marked rise in intraocular pressure.) Intraocular pressure is then controlled medically if possible, but the extent of peripheral anterior synechia formation and sluggish outflow through the remaining trabecular meshwork make pressure control very difficult, so that drainage surgery is often required. Epinephrine and strong miotics must not be used unless peripheral iridectomy has been performed because they will accentuate angle closure.
PLATEAU IRIS
Plateau iris is an uncommon condition in which the central anterior chamber depth is normal but the anterior chamber angle is very narrow owing to a congenital high insertion of the iris. Such an eye has little pupillary block, but dilation will cause bunching up of the peripheral iris, occluding the angle (angle crowding) even if peripheral iridectomy has been performed. Affected individuals present with acute angle-closure glaucoma at a young age, with recurrences after peripheral iridectomy. Long-term miotic therapy or laser iridoplasty is required.
Pupillary dilation for fundus examination is apt to cause acute angle closure in patients with plateau iris and may precipitate a similar event in other eyes with deep anterior chambers-due to angle crowding rather than the pupillary block mechanism seen in eyes with shallow anterior chambers.
CONGENITAL GLAUCOMA
Congenital glaucoma (rare) can be subdivided into (1) primary congenital glaucoma, in which the developmental abnormalities are restricted to the anterior chamber angle; (2) the anterior segment developmental anomalies-Axenfeld's syndrome, Peter's anomaly, and Rieger's syndrome-in which iris and corneal development are also abnormal; and (3) a variety of other conditions-including aniridia, Sturge-Weber syndrome, neurofibromatosis-1, Lowe's syndrome, and congenital rubella-in which the developmental anomalies of the angle are associated with other ocular or extraocular abnormalities.
Clinical Findings
Congenital glaucoma is manifest at birth in 50%, diagnosed in the first 6 months in 70%, and diagnosed by the end of the first year in 80%. The earliest and most common symptom is epiphora. Photophobia and decreased corneal luster may be present. Increased intraocular pressure is the cardinal sign. Glaucomatous cupping of the optic disk is a relatively early-and the most important-change. Later findings include increased corneal diameter (above 11.5 mm is considered significant), epithelial edema, tears of Des-cemet's membrane, and increased depth of the anterior chamber (associated with general enlargement of the anterior segment of the eye) as well as edema and opacity of the corneal stroma (Figure 11-9).
Differential Diagnosis
Megalocornea, corneal clouding due to congenital dystrophy or mucopolysaccharidoses, and traumatic rupture of Descemet's membrane should be ruled out. Measurement of intraocular pressure, gonioscopy, and evaluation of the optic disk are important in making the differential diagnosis. Assessment generally requires examination under general anesthesia.
Course & Prognosis
In untreated cases, blindness occurs early. The eye undergoes marked stretching and may even rupture with minor trauma. Typical glaucomatous cupping occurs relatively soon, emphasizing the need for early treatment.
1. ANTERIOR SEGMENT DEVELOPMENTAL ANOMALIES
These rare diseases constitute a spectrum of maldevelopment of the anterior segment, involving the angle, iris, cornea, and occasionally the lens. Usually there is some hypoplasia of the anterior stroma of the iris, with bridging filaments connecting the iris stroma to the cornea. If these bridging filaments occur peripherally and connect to a prominent, axially displaced Schwalbe's line (posterior embryotoxon), the disease is known as Axenfeld's syndrome. This resembles the trabeculodysgenesis of primary congenital glaucoma. If there are broader iridocorneal adhesions associated with the disruption of the iris, with polycoria and, in addition, skeletal and dental anomalies, the disorder is called Rieger's syndrome (an example of iridotrabecular dysgenesis). If adhesions are between the central iris and the central posterior surface of the cornea, the disease is known as Peter's anomaly (an example of iridocorneal trabeculodysgenesis).
These diseases are usually dominantly inherited, though sporadic cases have been reported. Mutations on chromosomes 4 and 13, probably involving homeobox genes, have been identified in pedigrees with Axenfeld-Rieger syndrome. Glaucoma occurs in approximately 50% of such eyes and often does not present until late childhood or early adulthood. Goniotomy has a much lower success rate in these cases, and trabeculotomy or trabeculectomy may be recommended. Many such patients require long-term medical glaucoma therapy, and the prognosis is guarded for long-term retention of good visual function.
2. ANIRIDIA
The distinguishing feature of aniridia, as the name implies, is the vestigial iris. In many cases, little more than the root of the iris or a thin iris margin is present. Other deformities of the eye may be present, such as congenital cataracts, corneal dystrophy, and foveal hypoplasia. Vision is usually poor. Glaucoma frequently develops before adolescence and is usually refractory to medical or surgical management.
This rare syndrome is genetically determined. Both autosomal dominant and autosomal recessive inheritance have been reported.
If medical therapy is ineffective, goniotomy or trabeculotomy may occasionally normalize the intraocular pressure. Filtering operations are often necessary, but the long-term visual prognosis is poor.
SECONDARY GLAUCOMA
Increased intraocular pressure occurring as one manifestation of some other eye disease is called secondary glaucoma. These diseases are difficult to classify satisfactorily. Treatment involves controlling intraocular pressure by medical and surgical means but also dealing with the underlying disease if possible.
PIGMENTARY GLAUCOMA
This syndrome seems to be primarily a degeneration of the pigmented epithelium of the iris and ciliary body. The pigment granules flake off from the iris as a result of friction against the underlying packets of zonular fibers, resulting in iris transillumination. The pigment is deposited on the posterior corneal surface (Krukenberg's spindle) and becomes lodged in the trabecular meshwork, impeding the normal outflow of aqueous. The syndrome occurs most often in myopic males between the ages of 25 and 40 who have a deep anterior chamber with a wide anterior chamber angle.
The pigmentary changes may be present without glaucoma (pigment dispersion syndrome), but such persons must be considered "glaucoma suspects." A number of pedigrees of autosomal dominant inheritance of pigmentary glaucoma have been reported, and a gene for pigment dispersion syndrome has been mapped to chromosome 7.
The logical treatment in this condition is miotic therapy because it overcomes movement of the iris across the zonules. However, because the patients are usually young myopes, such therapy is poorly tolerated unless administered as pilocarpine once daily, preferably at bedtime. Beta-blockers and epinephrine are also effective.
The major problem, however, is the young age at which the disease develops, which increases the chance that drainage surgery will be necessary and enhances the advisability of combining such surgery with antimetabolite therapy. Laser trabeculoplasty is frequently used in this condition but is unlikely to obviate the need for drainage surgery.
EXFOLIATION SYNDROME (Pseudo-Exfoliation Syndrome)
In exfoliation syndrome, flake-like deposits of a fibrillary material are seen on the anterior lens surface (in contrast to the true exfoliation of the lens capsule caused by exposure to infrared radiation, ie, "glassblower's cataract"), ciliary processes, zonule, posterior iris surface, loose in the anterior chamber, and in the trabecular meshwork (along with increased pigmentation). These deposits can also be detected histologically in the conjunctiva, suggesting a more widespread abnormality. The disease usually occurs in patients over age 65. Treatment is as for primary open-angle glaucoma.
GLAUCOMA SECONDARY TO CHANGES IN THE LENS
Lens Dislocation
The crystalline lens may be dislocated as a result of trauma or spontaneously, as in Marfan's syndrome. Anterior dislocation may cause obstruction of the pupillary aperture, leading to iris bombé and angle closure. Posterior dislocation into the vitreous is also associated with glaucoma, though the mechanism is obscure. It may be due to angle damage at the time of traumatic dislocation.
In anterior dislocation, the definitive treatment is lens extraction once the intraocular pressure has been controlled medically. In posterior dislocation, the lens is usually left alone and the glaucoma treated as primary open-angle glaucoma.
Intumescence of the Lens
The lens may take up considerable fluid during cataractous change, increasing markedly in size. It may then encroach upon the anterior chamber, producing both pupillary block and angle crowding and resulting in angle-closure glaucoma. Treatment consists of lens extraction once the intraocular pressure has been controlled medically.
Phacolytic Glaucoma
Some advanced cataracts may develop leakiness of the anterior lens capsule, which allows passage of liquefied lens proteins into the anterior chamber. There is an inflammatory reaction in the anterior chamber, and the trabecular meshwork becomes edematous and obstructed with lens proteins, leading to an acute rise in intraocular pressure. Lens extraction is the definitive treatment once the intraocular pressure has been controlled medically and intensive topical steroid therapy has reduced the intraocular inflammation.
GLAUCOMA SECONDARY TO CHANGES IN THE UVEAL TRACT
Uveitis
The intraocular pressure is usually below normal in uveitis because the inflamed ciliary body is functioning poorly. However, elevation of intraocular pressure may also occur through a number of different mechanisms. The trabecular meshwork may become blocked by inflammatory cells from the anterior chamber, with secondary edema, or may occasionally be involved in an inflammatory process specifically directed at the trabecular cells (trabeculitis). Chronic or recurrent uveitis produces permanent impairment of trabecular function, peripheral anterior synechiae, and occasionally angle neovascularization, all of which increase the chance of secondary glaucoma. Seclusio pupillae due to 360-degree posterior synechiae produces iris bombé and acute angle-closure glaucoma. The uveitis syndromes that tend to be associated with secondary glaucoma are Fuchs's heterochromic cyclitis, HLA-B27-associated acute anterior uveitis, and uveitis due to herpes zoster and herpes simplex.
Treatment is directed chiefly at controlling the uveitis with concomitant medical glaucoma therapy as necessary, avoiding miotics because of the increased chance of posterior synechia formation. Long-term therapy, including surgery, is often required because of irreversible damage to the trabecular meshwork.
Acute angle closure due to seclusion of the pupil may be reversed by intensive mydriasis but often requires laser peripheral iridotomy or surgical iridectomy. Any uveitis with a tendency to posterior synechia formation must be treated with mydriatics whenever the uveitis is active to reduce the risk of pupillary seclusion.
Tumor
Uveal tract melanomas may cause glaucoma by anterior displacement of the ciliary body, causing secondary angle closure, direct involvement of the anterior chamber angle, blockage of the filtration angle by pigment dispersion, and angle neovascularization. Enucleation is usually necessary.
IRIDOCORNEOENDOTHELIAL (ICE) SYNDROME (Essential Iris Atrophy, Chandler's Syndrome, Iris Nevus Syndrome)
This rare idiopathic condition of young adults is usually unilateral and manifested by corneal decompensation, glaucoma, and iris abnormalities.
GLAUCOMA SECONDARY TO TRAUMA
Contusion injuries of the globe may be associated with an early rise in intraocular pressure due to bleeding into the anterior chamber (hyphema). Free blood blocks the trabecular meshwork, which is also rendered edematous by the injury. Treatment is initially medical, but surgery may be required if the pressure remains elevated.
Late effects of contusion injuries on intraocular pressure are due to direct angle damage. The interval between the injury and the development of glaucoma may obscure the association. Clinically, the anterior chamber is seen to be deeper than in the fellow eye, and gonioscopy shows recession of the angle. Medical therapy is usually effective, but drainage surgery may be required.
Laceration or contusional rupture of the anterior segment is associated with loss of the anterior chamber. If the chamber is not reformed soon after the injury-either spontaneously, by iris incarceration into the wound, or surgically-peripheral anterior syne-chiae will form and result in irreversible angle closure.
GLAUCOMA FOLLOWING OCULAR SURGERY
Ciliary Block Glaucoma (Malignant Glaucoma)
Surgery upon an eye with markedly increased intraocular pressure and a closed angle can lead to ciliary block glaucoma. Immediately after surgery, the intraocular pressure increases markedly, and the lens is pushed forward as a result of the collection of aqueous in and behind the vitreous body.
Treatment consists of cycloplegics, mydriatics, aqueous suppressants, and hyperosmotic agents. Hyperosmotic agents are used to shrink the vitreous body and let the lens fall more posteriorly.
Posterior sclerotomy, vitrectomy, and even lens extraction may be needed.
Peripheral Anterior Synechiae
Just as with trauma to the anterior segment (see above), surgery that results in a flat anterior chamber will lead to formation of peripheral anterior synechiae. Early surgical re-formation of the chamber is required if it does not occur spontaneously.
NEOVASCULAR GLAUCOMA
Neovascularization of the iris (rubeosis iridis) and anterior chamber angle is most often secondary to widespread retinal ischemia such as occurs in advanced diabetic retinopathy and ischemic central retinal vein occlusion. Glaucoma results initially from obstruction of the angle by the fibrovascular membrane, but subsequent contraction of the membrane leads to angle closure.
Treatment of established neovascular glaucoma is difficult and often unsatisfactory.
GLAUCOMA SECONDARY TO RAISED EPISCLERAL VENOUS PRESSURE
Raised episcleral venous pressure may contribute to glaucoma in Sturge-Weber syndrome, in which a developmental anomaly of the angle is also often present, and carotid-cavernous fistula, which may also cause angle neovascularization due to widespread ocular ischemia. Medical treatment cannot reduce the intraocular pressure below the level of the abnormally elevated episcleral venous pressure, and surgery is associated with a high risk of complications.
STEROID-INDUCED GLAUCOMA
Topical and periocular corticosteroids may produce a type of glaucoma that simulates primary open-angle glaucoma, particularly in individuals with a family history of the disease, and will exaggerate the intraocular pressure elevation in those with established primary open-angle glaucoma. Withdrawal of the medication usually eliminates these effects, but permanent damage can occur if the situation goes unrecognized too long. If topical steroid therapy is absolutely necessary, medical glaucoma therapy will usually control the intraocular pressure. Systemic steroid therapy is less likely to cause a rise in intraocular pressure.
It is imperative that patients receiving topical or systemic steroid therapy undergo periodic tonometry and ophthalmoscopy, particularly if there is a family history of glaucoma.
REFERENCESList of Figures
| Figure 11-1: Anterior segment structures. Arrows indicate direction of flow of aqueous. | |
| Figure 11-2: Equal-pressure balloons. | |
| Figure 11-3: Composite illustration showing anatomic (left) and gonioscopic (right) view of normal anterior chamber angle. (Courtesy of R Shaffer.) | |
| Figure 11-4: Estimation of depth of anterior chamber by oblique illumination (diagram). (Courtesy of R Shaffer.) | |
| Figure 11-5: Typical glaucomatous cupping. Note the nasal displacement of the vessels and hollowed-out appearance of the optic disk except for a thin border. (Courtesy of S Mettier Jr.) | |
| Figure 11-6: Cross-section of an eye with open-angle glaucoma. Note open anterior chamber angle (peripheral iris is not in contact with the posterior corneal surface). Deep glaucomatous cupping ("bean-pot" appearance) shows the process to be well advanced. (Courtesy of R Carriker.) | |
| Figure 11-7: Glaucomatous ("bean-pot") cupping of the optic disk. | |
| Figure 11-8: Visual field changes in glaucoma. (Reproduced, with permission, from Harrington DO: The Visual Fields: A Textbook and Atlas of Clinical Perimetry, 5th ed. Mosby, 1981.) | |
| Figure 11-9: Congenital glaucoma (buphthalmos.) |
List of Tables
| Table 11-1: Glaucoma classified according to etiology. | |
| Table 11-2: Glaucoma classified according to mechanism of intraocular pressure rise. |
10.1036/1535-8860.ch11