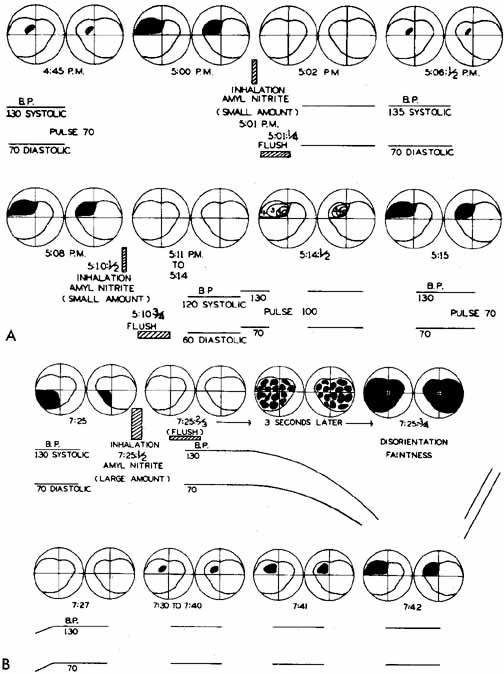
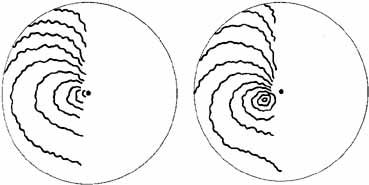
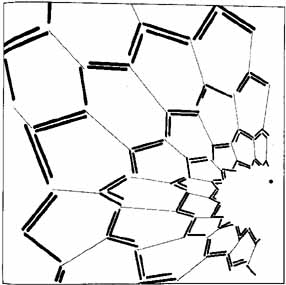
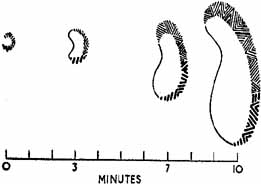
1. The International Classification of Headache Disorders. Published on behalf
of the International Headache Society. Cephalalgia 24(Suppl 1):1, 2004 2. Lipton RB, Stewart WF: Migraine in the United States: epidemiology and health care utilization. Neurology 43(Suppl 3):6, 1993 3. Stewart WF, Lipton RB, Celentano DD, et al: Prevalence of migraine headache in the United States. JAMA 267:64, 1992 4. Rasmussen BK, Jensen R, Schroll M: Epidemiology of headache in a general population: a prevalence study. J Clin Epidemiol 44:1147, 1991 5. Sacks OW: Migraine: The Evolution of a Common Disorder. Los Angeles: University of California Press, 1970 6. Gowers WR: Subjective visual sensations. Trans Ophthalmol Soc UK 15:1, 1895 7. Troost BT, Newton TH: Occipital lobe arteriovenous malformations: clinical and radiologic features
in 26 cases with comments on the differentiation from migraine. Arch Ophthalmol 93:250, 1975 8. Blau JN: Migraine prodromes separated from the aura: complete migraine. Br Med J 281:658, 1980 9. Silberstein SD, Lipton RB: Overview of diagnosis and treatment of migraine. Neurology 44(Suppl 7):6, 1994 10. Lipton RB, Stewart W, Celentano DD, et al: Undiagnosed migraine: a comparison of symptom-based and self-reported
physician diagnosis. Arch Intern Med 156:1, 1992 11. Stewart WF, Schecter A, Lipton RB: Migraine heterogenity: disability, pain intensity, attack frequency, and
duration. Neurology 44(Suppl 4):24, 1994 12. Amery WK, Van Neueten JM, Wauquier A: The Pharmocological Basis of Migraine Therapy. London: Pitman, 1984 13. Rose CF: Progress in Migraine Research 2. London: Pitman, 1984 14. Raskin NH: Headache, 2 ed. New York: Churchill Livingstone, 1988 15. Dalessio DJ: Wolff's Headache and Other Head Pain, 6 ed. New York: Oxford University Press, 1993 16. Goadsby PJ, Lipton RB, Ferrari MD: Migraine: current understanding and treatment. N Engl J Med 346:257, 2002 17. Goadsby PJ: Pathophysiology of headache. In: Silberstein SD, Lipton RB, Dalessio DJ, eds. Wolff's Headache and Other Pain, 7th ed. Oxford, England: Oxford University Press, 2001:57–72 18. Ophoff RA, Terwindt GM, Vergouwe MN, et al: Familial hemiplegic migraine and episodic ataxia type–2 are caused
by mutations in the Ca2+ channel gene CACNL1A4. Cell 1996;87:543–52. 19. Terwindt GM, Ophoff RA, van Eijk R, et al: Involvement of the CACNA1A gene containing region on 19p13 in migraine
with and without aura. Neurology 56:1028, 2001 20. Kors E, Haan J, Ferrari M: Migraine genetics. Curr Headache Rep 2:72, 2003 21. Russell MB, Olesen J: Increased familial risk and evidence of genetic factor in migraine. BMJ 311:541, 1995 22. Wessman M, Kallela M, Kaunisto MA, et al: A susceptibility locus for migraine with aura, on chromosome. Am J Hum Genet 70:652, 2002 23. Welch KMA: Relationship of stroke and migraine. Neurology 44(Suppl 7):33, 1994 24. Olesen J, Larsen B, Lautitzen M: Focal hyperemia followed by spreading oligemia and impaired activation
of rCBF in classic migraine. Ann Neurol 9:344, 1981 25. Skyhoj-Olesen TS, Friberg L, Lassen NA: Ischemia may be the primary cause of the neurologic deficits in classic
migraine. Arch Neurol 44:156, 1987 26. May A, Goadsby PJ: The trigeminovascular system in humans: pathophysiologic implications for
primary headache syndromes of the neural influences on the cerebral
circulation. J Cereb Blood Flow Metab 19:115, 1999 27. Weiller C, May A, Limmroth V, et al: Brain stem activation in spontaneous human migraine attacks. Nat Med 1:658, 1995 28. Bahra A, Matharu MS, Buchel C, et al: Brainstem activation specific to migraine headache. Lancet 357:1016, 2001 29. Sicuteri F: Vasoneuroactive substances in migraine. Headache 6:109, 1966 30. Lance JW, Anthony M, Gonski A: Serotonin, the carotid body, and cranial vessels in migraine. Arch Neurol 16:553, 1067 31. Curran AC, Hinterberger H, Lance JW: Total plasma serotonin, 5-hydroxyindoleacetic acid and p-hydroxy-m-methoxymandelic acid excretion in normal and migrainous
subjects. Brain 88:997, 1966 32. Moskowitz MA: The neurobiology of vascular head pain. Ann Neurol 16:157, 1984 33. Raskin NH: Chemical headaches. Ann Rev Med 32:63, 1981 34. Wolff HG: Headache and Other Head Pain. New York: Oxford University Press, 1963 35. O'Brien M: Cerebral blood changes in migraine. Headache 10:139, 1971 36. Simard D, Pauson OB: Cerebral vasomotor paralysis during migraine attack. Arch Neurol 29:207, 1973 37. Brewerton TD, Murphy DL, Mueller EA, et al: Induction of migraine-like headaches by the serotonin agonist m-chlorophenylpiperazine. Clin Pharmacol Ther 43:605, 1988 38. Gordon ML, Lipton RB, Brown SL, et al: Headache and cortical responses to m-chlorophenylpiperazine are
highly correlated. Cephalalgia 13:400, 1993 39. Friedman AP, Merritt HH: Headache: Diagnosis and Treatment. Philadelphia: Davis, 1959 40. Callahan N: The migraine syndrome in pregnancy. Neurology 18:197, 1968 41. Shafey S, Scheinberg P: Neurological syndromes in patients receiving synthetic steroids. Neurology 16:205, 1966 42. Phillips BM: Oral contraceptive drugs and migraine. Br Med J 2:99, 1968 43. Whitty CWM, Hockaday KM, Whitty MM: The effect of oral contraceptives on migraine. Lancet 1:856, 1966 44. Somerville BW: Estrogen-withdrawal migraine: I, Duration or exposure required and
attempted prophylaxis by premenstrual estrogen administration. Neurology 25:235, 1975 45. Hanington E, Horn M, Wilkinson M: Further observations on the effects of tyramine. In: Cochrane AL, ed. Background to Migraine, Third Migraine Symposium. London: Heinemann, 1969:113–119 46. Haas DC, Pindela GS, Lourie H: Juvenile head trauma syndromes and their relationship to migraine. Arch Neurol 32:727, 1975 47. Paulson GW, Klawans HL: Benign orgasmic cephalgia. Headache 13:181, 1974 48. Milner PM: Note on a possible correspondence between the scotomas of migraine and
spreading depression of Lea. Electroencephalogr Clin Neurophysiol Suppl 10:705, 1956 49. Lashley KS: Patterns of cerebral integration indicated by scotomas of migraine. Arch Neurol Psych 46:333, 1941 50. Leao AAP: Spreading depression of activity in the cerebral cortex. J Neurophysiol 7:359, 1944 51. Woods RP, Iacoboni M, Mazziotta JC: Bilateral spreading cerebral hypoperfusion during spontaneous migraine
headache. N Engl J Med 331:1689, 1994 52. Sanchez Del Rio M, Bakker D, Wu O, et al: Perfusion weighted imaging during migraine: spontaneous visual aura and
headache. Cephalalgia 19:701, 1999 53. Cutrer FM, Sorensen AG, Weisskoff RM, et al: Perfusion-weighted imaging defects during spontaneous migrainous
aura. Ann Neurol 43:25, 1998 54. Lauritzen M: Pathophysiology of the migraine aura: the spreading depression theory. Brain 117:199, 1994 55. Hadjikhani N, Sanchez Del Rio M, Wu O, et al: Mechanisms of migraine aura revealed by functional MRI in human visual
cortex. Proc Natl Acad Sci USA 98:4687, 2001 56. Olesen J, Friberg L, Olsen TS, et al: Timing and topography of cerebral blood flow, aura, and headache during
migraine attacks. Ann Neurol 28:791, 1990 57. Cao Y, Welch KMA, Aurora S, et al: Functional MRI-BOLD of visually triggered headache in patients with
migraine. Arch Neurol 56:548, 1999 58. Feindel W, Penfield W, McNaughton F: The tentorial nerves and localization of intracranial pain in man. Neurology 10:555, 1960 59. Arbab MA-R, Wiklund L, Svendgaard NA: Origin and distribution of cerebral vascular innervation from superior
cervical, trigeminal, and spinal ganglia investigated with retrograde
and anterograde WGA-HRP tracing in the rat. Neuroscience 19:695, 1986 60. Goadsby PJ, Edvinsson L, Ekman R: Vasoactive peptide release in the extracerebral circulation of humans during
migraine headache. Ann Neurol 28:183, 1990 61. Moskowitz MA, Cutrer FM: Sumatriptan: a receptor-targeted treatment for migraine. Ann Rev Med 44:145, 1993 62. Troost BT: Botulinum toxin type A (BoNT-A) in the management of headache: a
review of the literature and personal experience. J Headache Pain 5:15–22, 2004 63. Burstein R, Yarnitsky D, Goor-Aryeh I, et al: An association between migraine and cutaneous allodynia. Ann Neurol 47:614, 2000 64. May A, Buchel C, Turner R, et al: Magnetic resonance angiography in facial and other pain: neurovascular
mechanisms of trigeminal sensation. J Cereb Blood Flow Metab 21:1171, 2001 65. Alverez WC: The migrainous scotoma is studied in 618 persons. Am J Ophthalmol 49:389, 1960 66. Klee A, Willanger R: Disturbance of visual perception in migraine. Acta Neurol Scand 42:400, 1966 67. Walsh FB, Hoyt WF: Clinical Neuro-Ophthalmology, 3rd ed. Baltimore: Williams & Wilkins, 1969. 68. Gowers WR: Visual sensations in migraine. In: Gowers WR, ed. Subjective Sensations of Sight and Sound, Abiotrophy and Other Lectures. London: Churchill, 1907:18–41. 69. Peatfield C, Rose F: Migrainous visual symptoms in a woman without eyes. Arch Neurol 38:466, 1981 70. Day JW, Raskin NH: Thunderclap headache: symptoms of unruptured aneursym. Lancet 2:1247, 1986 71. Haas DC: Prolonged migraine aura status. Ann Neurol 11:197, 1982 72. Fisher CM: Late-life migraine accompaniments as a cause of unexplained transient
ischemic attacks. Can J Neurol Sci 7:9, 1980 73. Troost BT, Mark LE, Maroon JC: Resolution of classic migraine following removal of an occipital lobe arteriovenous
malformation. Ann Neurol 5:199, 1979 74. Bartleson JD: Transient and persistent neurological manifestations of migraine. Stroke 15:383, 1984 75. Berg MJ, Williams LS: The transient syndrome of headache with neurologic deficits and CSF lymphocytosis. Neurology 45:1648, 1995 76. Dvorkin GS, Andermann F, Carpenter S, et al: Classical migraine, intractable epilepsy, and multiple strokes: a syndrome
related to mitochondrial encephalomyopathy. In: Andermann F, Lugaresi E, eds. Migraine and Epilepsy. Stoneham, MA.: Butterworths, 1987:203–232. 77. Fitzsimons R, Wolfenden WH: Migraine coma: menigitic migraine with cerebral edema associated with a
new form of autosomal dominant cerebellar ataxia. Brain 108:555, 1985 78. Caplan L: Intracerebral hemorrhage revisited. Neurology 38:624, 1988 79. Levine SR, Joseph R, D'Andrea G, et al: Migraine and the lupus anticoagulant. Cephalalgia 7:93, 1987 80. Ormond AW: Two cases of permanent hemianopsia following severe attacks of migraine. Ophthalmol Rev 32:192, 1913 81. Butler TH: Scotoma in migrainous subjects. Br J Ophthalmol 17:83, 1933 82. Butler TH: Uncommon symptoms of migraine. Trans Ophthalmol Soc UK 61:205, 1941 83. Rothrock JF, Walicke P, Swenson MR, et al: Migrainous stroke. Arch Neurol 45:63, 1988 84. Bougousslavsky J, Regli F, VanMelle G, et al: Migraine stroke. Neurology 38:223, 1988 85. Tzourio C, Iglesias S, Hubert J-B, et al: Migraine and risk of ischemic stroke: a case-controlled study. Br Med J 307:289, 1993 86. Hollenhorst RB: Ocular manifestations of migraine: Report of 4 cases of hemianopsia. Proc Staff Meeting Mayo Clinic 28:686, 1958 87. Rich WM: Permanent quadrantanopia after migraine. Br Med J 116:592, 1948 88. Bruyn GW: Complicated migraine. In: Vinken PJ, Bruyn GW, eds. Handbook of Clinical Neurology. New York: American Elsevier, 1968:59–95. 89. Connor RCR: Complicated migraine: a study of permanent neurological and visual defects
by migraine. Lancet 2:1072, 1962 90. Caplan L, Chedru F, Lhermitte F, et al: Transient global amnesia and migraine. Neurology 31:1167, 1981 91. Selby G, Lance JW: Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psych 23:23, 1960 92. Smyth VOG, Winter AL: The EEG and migraine. Electroencephalogr Clin Neurophysiol 16:194, 1964 93. Boudin G, Pepin B, Barbizet J, et al: Migraine and EEG disturbances. Electroencephalogr Clin Neurophysiol 14:141, 1962 94. Hockaday JM, Whitty CWM: Factors determining the electroencephalogram in migraine: a study of 560 patients
according to clinical type of migraine. Brain 92:769, 1969 95. Friedman MW: Occlusion of central retinal vein in migraine. Arch Ophthalmol 45:678, 1951 96. Heyck H: Pathogenesis of migraine. In: Friedman AP, ed. Research and Clinical Studies in Headache. New York: Karger, 1969:1–28. 97. Heyck H: Varieties of hemiplegic migraine. Heachache 14:135, 1973 98. Alpers BJ, Yaskin HE: Pathogenesis of ophthalmoplegic migraine. Arch Ophthalmol 45:555, 1951 99. Patterson RH, Goodell H, Dunning HS: Complications of carotid arteriography. Arch Neurol 10:513, 1964 100. Friedman AP, Finley KH, Graham JR, et al: Classification of headache. Neurology 12:173, 1962 101. Whitty CWM: Familial hemiplegic migraine. J Neurol Neurosurg Psych 16:172, 1953 102. Blau JN, Whitty CWM: Familial hemiplegic migraine. Lancet 2:1115, 1955 103. Heyck H: Die neurologischen Begleiterscheinungen der Migraine und das Problem des “angiospastischen
Herninsults.” Nervenarzt 33:193, 1962 104. Rosenbaum HE: Familial hemiplegic migraine. Neurology 10:164, 1960 105. Ohta M, Araki S, Kuroiwa Y: Familial occurrence of migraine with hemiplegic syndrome and cerebellar
manifestations. Neurology 17:813, 1967 106. Young GF, Leon-Barth CA, Green J: Familial hemiplegic migraine, retinal degeneration, deafness, and nystagmus. Arch Neurol 23:201, 1970 107. Dooling EC, Sweeney VP: Migrainous hemiplegia during breast feeding. Am J Obstet Gynecol 118:568, 1974 108. Straube A, Bandmann O, Buttner U, et al: A contrast enhanced lesion of the III nerve on MR of a patient with ophthalmoplegic
migraine as evidence for a Tolosa-Hunt syndrome. Headache 33:446, 1994 109. Wong V, Wong WC: Enhancement of oculomotor nerve: a diagnostic criterion for ophthalmoplegic
migraine. Pediatr Neurol 17:70, 1997 110. Troost BT: Migraine, ophthalmoplegic and retinal. In: Arminoff M, Daroff RB, eds. Encyclopedia of the Neurological Sciences Vol 3. San Diego, CA: Academic Press, 2003:167 111. Troost BT, Tomsak RL: Ophthalmoplegic migraine and retinal nigraine. In: Olesen J, Tfelt-Hansen P, Welch KMA, eds. The Headaches. New York: Raven Press Ltd., 1993:421–426. 112. Tomsak RL, Masaryk TJ, Bates JH: Magnetic resonance angiography (MRA) of isolated aneurysmal third
nerve palsy. J Clin Neuroophthalmol 11:16, 1991 113. Woody RC, Blaw ME: Ophthalmoplegic migraine in infancy. Clin Pediatr 25:82, 1986 114. Hallet M, Cogan DG: Episodic unilateral mydriasis in otherwise normal patients. Arch Ophthalmol 84:130, 1970 115. Edelson RN, Levy DE: Transient benign unilateral pupillary dilatation in young adults. Arch Neurol 31:12, 1974 116. Sarkies NJC, Sanders MD, Gautier-Smith PC: Episodic unilateral mydriasis and migraine [letter]. Am J Ophthalmol 99:217, 1985 117. Durkan GP, Troost BT, Slamovits TL, et al: Recurrent painless oculomotor palsy in children: a variant of ophthalmoplegic
migraine. Headache 21:58, 1981 118. Gabianelli EB, Klingele TG, Burde RM: Acute oculomotor nerve palsy in childhood. Is arteriography necessary? J Clin Neuroophthalmol 9:33, 1989 119. Walsh JP, O'Doherty DS: A possible explanation of the mechanism of ophthalmoplegic migraine. Neurology 10:1079, 1960 120. Friedman AP, Harter DH, Merritt HH: Ophthalmoplegic migraine. Arch Neurol 7:320, 1962 121. Osuntoken O, Osuntoken BO: Ophthalmoplegic migraine and hemoglobinopthy in Nigerians. Am J Ophthalmol 74:451, 1972 122. Anderson DR: Perimetry: With and without Automation, 2nd ed. St. Louis: C.V. Mosby, 1987 123. Tomsak RL, Jergens PB: Benign recurrent transient monocular blindness: a possible variant of acephalgia
migraine. Headache 27:66, 1987 124. Kline LB, Kelley CL: Ocular migraine in a patient with cluster headache. Headache 20:253, 1980 125. Gronvall A: On changes in the fundus oculi and persisting injuries to the eye in migraine. Acta Ophthalmol 16:602, 1938 126. Cassen JH, Tomsak RL, DeLuise VP: Mixed arteriovenous occlusive disease of the fundus. J Clin Neuroophthalmol 5:164, 1985 127. Wolter JR, Burchfield WJ: Ocular migraine in a young man resulting in unilateral transient blindness
and retinal edema. J Pediatr Ophthalmol 8:173, 1971 128. Burger SK, Saul RF, Selhorst JB, et al: Transient monocular blindness caused by vasospasm. N Engl J Med 325:870, 1991 129. Graveson GS: Retinal arterial occlusion in migraine. Br Med J 2:838, 1949 130. Krapin D: Occlusion of the central retinal artery in migraine. N Engl J Med 270:359, 1964 131. Dalessio DJ: Wolff's Headache and Other Head Pain, 4th ed. New York: Oxford University Press, 1980. 132. Hupp SL, Kline LB, Corbett JJ: Visual disturbance of migraine. Surv Ophthalmol 33:221, 1989 133. Newman NJ, Lessell S, Brandt M: Bilateral central retinal artery occlusions, disk drusen, and migraine. Am J Ophthalmol 107:236, 1989 134. Corbett JJ: Neuro-ophthalmic complications of migraine and cluster headaches. In: Smith CH, Beck RW, eds. Neurologic Clinics Symposium on Neuro-Ophthalmology. Philadelphia: WB Saunders, 1983:973–995. 135. Coppeto JR, Monteiro MLR, Sciarra R: Giant cell arteritis with bilateral uveitic glaucoma. Ann Ophthalmol 17:299, 1985 136. Lewis RA, Vijayan N, Watson C, et al: Visual field loss in migraine. Ophthalmology 96:321, 1989 137. Carroll D: Retinal migraine. Headache 10:9, 1970 138. Dirr LY, Janton FJ, Troost BT: Non-benign amaurosis fugax in a medical student. Neurology 40:349, 1990 139. McDonald WI, Sanders MD: Migraine complicated by ischemic papillopathy. Lancet 2:521, 1971 140. Laties AM: Central retinal artery innervation: Absence of adrenergic innervation to
the intraocular branches. Arch Ophthalmol 77:405, 1977 141. Coppeto JR, Lessell S, Sciarra R, Bear L, Vascular retinopathy in migraine. Neurology 36(2):267–270, 1986 142. Larsen BH, Sorensen PS, Marquardsen J: Transient ischaemic attacks in young patients, a thromboembolic or migranous
minifestation? A ten year follow up of 46 patients. J Neurol Neurosurg Psychiatr 53:1029, 1990 143. Brown GC, Margargal LE, Shields JA, et al: Retinal arterial obstruction in children and young adults. Ophthalmology 88:18, 1981 144. Kupersmith MJ, Hass WK, Chase NE: Isoproterenol treatment of visual symptoms in migraine. Stroke 10:299, 1979 145. Kuhn WF, Kuhn SC, Daylida L: Basilar migraine. Eur J Emerg Med 4:33, 1997 146. Panayiotopoulos CP: Basilar migraine. Neurology 41:1707, 1991 147. Bickerstaff ER: Basilar artery migraine. Lancet 1:15, 1961 148. Bickerstaff ER: Impairment of consciousness in migraine. Lancet 2:1057, 1961 149. Lees F, Watkins SM: Loss of consciousness in migraine. Lancet 186:647, 1963 150. Basser LS: The relation of migraine and epilepsy. Brain 92:285, 1969 151. Bickerstaff ER: The basilar artery and the migraine-epilepsy syndrome. Proc R Soc Med 55:167, 1962 152. Guest IA, Woolf AL: Fatal infarction of the brain in migraine. Br Med J 1:225, 1964 153. Swanson JW, Vick NA: Basilar artery migraine, 12 patients, with an attack recorded electroencephalographically. Neurology 28:782, 1978 154. Lance JW: The Mechanisms and Managment of Headache. London: Butterworth, 1969. 155. Whitty CWM: Migraine variants. Br Med J 1:38, 1971 156. Gascon G, Barlow C: Juvenile migraine, presenting as an acute confusional state. Pediatrics 45:628, 1970 157. Whitty CWM: Migraine without headache. Lancet 2:283, 1967 158. Bigal ME, Sheftell FD, Rapoport AM, et al: Chronic daily headache in a tertiary care population: correlation between
the International Headache Society diagnostic criteria and proposed
revisions of criteria for chronic daily headache. Cephalalgia 22:432, 2002 159. Scher AI, Stewart WF, Liberman J, et al: Prevalence of frequent headache in a population sample. Headache 38:497, 1998 160. Mathew NT, Kurman R, Perez F: Drug induced refractory headache—clinical features and management. Headache 30:634, 1990 161. Silberstein SD, Lipton RB: Chronic daily headache. In: Goadsby PJ, Silberstein SD, eds. In Headache. Newton: Butterworth-Heinemann, 1997:201–225. 162. Kuncle EC, Anderson WB: Significance of minor eye signs in headaches of migraine type. Arch Ophthalmol 65:504, 1961 163. Bickerstaff EB: Cluster headaches. In: Vinken PJ, Bruyn GW, eds. Handbook of Clinical Neurology. New York: American Elsevier, 1968:111–118. 164. Eggleston DJ: Periodic migrainous neuralgia. Oral Surg 29:524, 1970 165. Nelson RF: Cluster Migraine: an unrecognized common entity. Can Med Assoc J 103:1026, 1970 166. Lance JW, Anthony M: Migrainous neuralgia or cluster headache. J Neurol Sci 13:401, 1971 167. Ekbom K: A clinical comparison of cluster headache and migraine. Acta Neurol Scand 46(Suppl 41):1, 1970 168. Romberg MH: A Manual of the Nervous Disease of Man. 1853:56 169. Harris W: Neuritis and Neuralgia. Oxford: Oxford University Press, 1926 170. Harris W: Ciliary (migrainous) neuralgia and its treatment. Br Med J 1:457, 1938 171. Ekbom K, Greitz T: Carotid angiography in cluster headache. Acta Radiol 10:177, 1970 172. Vail HH: Vidian neuralgia, with special reference to eye and orbital pain in suppuration
of petrous apex. Ann Otolaryngol 41:837, 1932 173. Aubry M, Pialoux P: Sluder's syndrome. In: Vinken PJ, Bruyn GW, eds. Handbook of Clinical Neurology. New York: American Elsevier, 1968:326–332. 174. Raeder JG: “Paratrigeminal” paralysis of oculopupillary sympathetic. Brain 47:149, 1924 175. Boniuk M, Schlezinger NS: Raeder's paratrigeminal syndrome. Am J Ophthalmol 54:1074, 1962 176. Ford FR, Walsh FB: Raeder's paratrigeminal syndrome: a benign disorder, possibly a complication
of migraine. Bull Johns Hopkins Hosp 103:296, 1958 177. Smith JL: Raeder's paratrigeminal syndrome. Am J Ophthalmol 46:194, 1958 178. Davis RH, Daroff RB, Hoyt WF: Hemicrania, oculosympathetic paresis, and subcranial carotic aneurysm: Raeder's
paratrigeminal syndrome (Group 2). J Neurosurg 29:94, 1968 179. Law WR, Nelson ER: Internal carotid aneurysm as a cause of Raeder's paratrigeminal syndrome. Neurology 18:43, 1968 180. Cohen DN, Zakov ZN, Salanga VD, et al: Raeder's paratrigeminal syndrome: a new etiology. Am J Ophthalmol 79:1044, 1975 181. Horton BT: Histaminic cephalgia: differential diagnosis and treatment. Proc Mayo Clin 31:325, 1956 182. Meyer JS, Binns PM, Ericsson AD, et al: Sphenopalatine ganglionectomy for cluster headache. Arch Otolaryngol 92:475, 1970 183. Anthony M, Lance JW: Histamine and serotonin in cluster headache. Arch Neurol 25:225, 1971 184. Graham JR: Methysergide for prevention of headache. N Engl J Med 270:67, 1964 185. Weber RB, Reinmuth OM: The treatment of migraine with propranolol. Neurology 22:366, 1972 186. Gelmers HJ: Nimodipine, a new calcium antagonist, in the prophylactic treatment of
migraine. Headache 23:106, 1983 187. Goadsby PJ, Lipton RB: A review of paroxysmal hemicranias, SUNCT syndrome and other short-lasting
headaches with autonomic features, including new cases. Brain 120:193, 1997 188. Goadsby PJ, Matharu MS, Boes CJ: SUNCT syndrome or trigeminal neuralgia with lacrimation. Cephalalgia 21:82, 2001 189. Penart A, Firth M, Bowen JRC: Short-lasting unilateral neuralgiform headache with conjunctival
injection and tearing (SUNCT) following presumed dorsolateral
brainstem infarction. Cephalalgia 21:236, 2001 190. Matharu MS, Levy MJ, Merry RT, et al: SUNCT syndrome secondary to prolactinoma. J Neurol Neurosurg Psych 74:1590, 2003 191. Vahlquist B: Migraine in children. Int Arch Allergy Appl Immunol 7:348, 1955 192. Michael MI, Williams JM: Migraine in children. J Pediatr 41:18, 1952 193. Krupp GR, Friedman AP: Recurrent headache in children: A study of 100 clinical cases. NY State J Med 53:43, 1933 194. Holguin J, Fenichel G: Migraine. J Pediatr 70:290, 1967 195. Hachinski VC, Porchawka J, Steele JC: Visual symptoms in the migraine syndrome. Neurology 23:570, 1973 196. Golden GS: The ‘Alice in Wonderland Syndrome’ in juvenile migraine. Pediatrics 63:517, 1979 197. Haas DC, Souner RD: Migraine attacks triggered by mild head trauma and their relation to certain
post-traumatic disorders of childhood. J Neurol Neurosurg Psych 32:548, 1969 198. Griffith JF, Dodge PR: Transient blindness following head injury in children. N Engl J Med 278:638, 1968 199. Greenblatt SH: Post-traumatic transient cerebral blindness: association with migraine
and seizure diathesis. JAMA 225:1073, 1973 200. Forsyth WI, Gillies D, Sills MA: Propanalol (‘Inderal’) in the treatment of childhood
migraine. Dev Med Child Neurol 26:737, 1984 201. Hinrichs WL, Keith HM: Migraine in childhood: a follow-up report. Mayo Clin Proc 40:593, 1965 202. Diamond S, Dalessio DJ: The Practicing Physician's Approach to Headache. Baltimore: Williams & Wilkins, 1986. 203. Olesen J, Tfelt-Hansen P, Welch KMA: The Headaches. New York: Raven Press, 1993 204. Troost BT: Migraine and facial pain. In: Lessell S, Van Dalen JTW, eds. Current Neuro-Ophthalmology. New York: Year Book Medical Publishers, 1988:269–287 205. Troost BT, McCormick GM: Migraine and facial pain. In: Lessell S, Van Dalen JTW, eds. Current Neuro-Ophthalmology. Chicago: Year Book Medical Publishers, 1989:269–287. 206. Swerdlow M: Anticonvulsant drugs and chronic pain. Clin Neuropharmacol 7:51, 1984 207. Janetta PJ: Microsurgical approach to the trigeminal nerve for tic douloureaux. Progr Neurol Surg 7:188, 1976 208. Janetta PJ: Microsurgical management of trigeminal neuralgia. Arch Neurol 42:800, 1985 209. Zorman G, Wilson CB: Outcome following microsurgical vascular decompression or partial sensory
rhizotomy in 125 cases of trigeminal neuralgia. Neurology 34:1362, 1984 210. Sweet WH: The treatment of trigeminal neuralgia. N Engl J Med 316:692, 1987 211. Allen NB, Studenski SA: Polymyalgia rheumatica and temporal arteritis. Med Clin North Am 79:369, 1986 212. Vilaseca J, Gonzalez A, Cid MC, et al: Clinical usefulness of temporal artery biopsy. Ann Rheum Dis 46:282, 1987 213. Soloman S, Cappa KG: The headache of temporal arteritis. J Am Geriatr Soc 35:163, 1987 214. Allen NB, Studenski SA: Polymyalgia rheumatica and temporal arteritis. Med Clin North Am 70:369, 1986 215. Currie J, Lessell S: Tonic pupil giant cell arteritis. Br J Ophthalmol 8:135, 1984 216. Monteiro MLR, Coppeto JR, Greco P: Giant cell arterities of the posterior cerebral circulation presenting
with ataxia and ophthalomoplegia. Arch Ophthalmol 102:407, 1984 217. Howard GF, HO SU, Kim KS, et al: Bilateral carotid artery occlusion resulting from giant cell arteritis. Ann Neurol 15:204, 1984 218. Truong L, Kopelman RG, Williams GS, et al: Temporal arteritis and renal disease. Am J Med 78:171, 1985 219. Perruquet JL, Davis DE, Harrington TM: Aortic arch arteritis in the elderly: an important manifestation of giant
cell arteritis. Arch Intern Med 146:289, 1986 220. Selsky IJ, Nirankari VS: Temporomandibular joint pain as a manifestation of temporal arteritis. So Med J 78:1249, 1985 221. Accetta DD, Kelley JF, Tubbs RR: An elderly black woman with a painful “swollen” face. Ann Allergy 55:819, 1985 222. Goodman BW, Shepard FA: Jaw claudication. Its value as a diagnostic clue. Postgrad Med 73:177, 1983 223. Save-Soderbergh J, Malmvall BE, Andersson R, et al: Giant cell arteritis as a cause of death: report of nine cases. JAMA 255:493, 1986 224. Wong RL, Korn JH: Temporal arteritis and normal sedimentation rates. Am J Med 80:959, 1986 225. Villalta J, Estrach T: Temporal arteritis with normal erythrocyte sedimentation [letter]. Ann Intern Med 103:808, 1988 226. Plum F: Temporal arteritis with normal sedimentation rate. Neurology Alert 4:46, 1986 227. Daroff RB: New headache classification. Neurology 38:1138, 1988 228. Ziegle DK: Tension-muscle contraction headaches. A review. In: Pfaffenrath V, Lundberg PO, Sjaastad O, eds. Updating Headache. Berlin: Springer-Verlag, 1985:315–320. 229. Jakobsen J, Baadsgaard SE, Thompsen S, et al: Prediction of post-concussional sequelae by reaction time test. Acta Neurol Scan 75:341, 1987 230. Leblanc R: The minor leak proceding subarachnoid hemorrahage. J Neurosurg 66:35, 1987 231. Mokri B, Sundt TM, Houser OW, et al: Spontaneous dissection of the cervical internal carotid artery. Ann Neurol 19:126, 1986 232. Carlow TJ: Headache and the eye. In: Dalessio DJ, ed. Wolff's Headache and Other Head Pain. New York: Oxford University Press, 1987:304–320 233. Vinken PJ, Bruyn GW: Handbook of Clinical Neurology. New York: American Elsevier, 1968 234. Francis KR, Williams DP, Troost BT: Facial numbness and dysesthesia. Arch Neurol 44:345, 1987 235. Dalessio DJ: Diagnosing the severe headache. Neurology 44(Suppl 3):6, 1994 236. Pincus JH, Daroff RB: Sphenoid sinus mucocele: A curable cause of the ophthalmoplegic migraine
syndrome. JAMA 187:459, 1964 237. Baumel B: Migraine: a pharmacologic review with newer options and delivery modalities. Neurology 44(Suppl 3):13, 1994 238. Edmeads J: Advances in migraine therapy: Focus on oral sumatriptan. Neurology 45(Suppl 7):3, 1995 239. Cutler N, Mushet GR, Davis R, et al: Oral sumatriptan for the acte treatment of migraine: Evaluation of three
dosage strengths. Neurology 45(Suppl 7):5, 1995 240. Sargent J, Kirchner JR, Davis R, et al: Oral sumatriptan is effective and well tolerated for the acute treatment
of migraine: results of a multicenter study. Neurology 45(Suppl 7):10, 1995 241. Rederich G, Rapoport A, Cutler N, et al: Oral sumatriptan for the long-term treatment of migraine: clinical
findings. Neurology 45(Suppl 7):15, 1995 242. Troost BT: Botulinum toxin type A (BoNT-A) in the treatment of migraine
and other headaches. Expert Rev Neurotherapeutics 2004:4(1);27–31 243. Blumenfeld A: Botulinum toxin type A as an effective prophylactic treatment in primary
headache disorders. Headache 43:853, 2003 244. Blumenfeld AM, Binder W, Silberstein SD, et al: Procedures for administering botulinum toxin type A for migraine and tension-type
headache. Headache 43:884, 2003 245. Ryan RE: A study of Midrin in the symptomatic relief of migraine headache. Headache 14:33, 1974 246. Tfelt-Hansen P, Lipton RB: Miscellaneous drugs. In: Olesen J, Tfelt-Hansen P, Welch KMA, eds. The Headaches. New York: Raven Press, 1993:353–358. 247. Humphrey PPA, Feniuk W, Perren MJ: Anti-migraine drugs in development: advances in serotonin receptor
pharmacology. Headache 30(Suppl 1):12, 1990 248. Peroutka SJ: Developments in 5-hydroxytryptamine receptor pharmacology in migraine. Neurol Clin 8:829, 1990 249. Peroutka SJ: The pharmacology of current antimigraine drugs. Headache 30(Suppl 1) 30:12, 1990 250. Callaham M, Raskin N: A controlled study of dihydroergotamine in the treatment of acute migraine
headache. Headache 26:168, 1986 251. Silberstein SD: Review: serotonin 5-HT and migraine. Headache 34:408, 1994 252. Silberstein SD, Saper J: Migraine: diagnosis and treatment. In: Dalessio D, Silberstein SD, eds. Wolff's Headache and Other Head Pain, 6th ed. New York: Oxford University Press, 1993:96–170 253. Wilkinson M: Migraine: treatment of acute attack. Br Med J 2:754, 1971 254. Goodman LS, Gilman A: The Pharmacological Basis of Therapeutics, 8th ed. New York: Pergamon Press, Inc., 1990 255. Raskin NH, Raskin KE: Repetitive intravenious dihydroergotamine for the treatment of intractable
migraine. Neurology 34(Suppl 1):245, 1984 256. Sanders SW, Haering N, Mosberg H, et al: Pharmacokinetics of ergotamine in healthy volunteers following oral and
rectal dosing. Eur J Clin Pharmacol 30:331, 1986 257. Volans GN: Research review: migraine and drug absorption. Clin Pharmacokinet 3:313, 1978 258. Sicuteri F, Franchi G, Del Bianco PL: An antaminic drug, BC–105, in the prophylaxis of migraine. Int Arch Allergy Appl Immunol 31:78, 1967 259. Tfelt-Hansen P, Saxena PR, Dahlof C, et al: Ergotamine in the acute treatment of migraine: a review and European consensus. Brain 123:9, 2000 260. Olesen J, Tfelt-Hansen P, Welch KMA: The Headaches, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2000 261. Welch KMA, Mathew NT, Stone P, et al: Tolerability of sumatriptan: clinical trials and post-marketing
experience. Cephalalgia 20:687, 2000 262. Goadsby PJ: The pharmacology of headache. Prog Neurobiol 62:509, 2000 263. Ferrari MD, Roon KI, Lipton RB, et al: Triptans (serotonin, 5-HT 1b/1D agonists) in acute
migraine treatment: a meta analysis of 53 trials. Lancet 358:1668, 2001 264. Goadsby PJ, Ferrari MD, Olesen J, et al: Eletriptan in acute migraine: a double-blind, placebo-controlled
comparison to sumatriptan. Eletriptan Steering Committee. Neurology 54:156, 2000 265. Mathew NT, Schoenen J, Winner P, et al: Compartive efficacy of eletriptan 40 mg versus sumatriptan 100 mg. Heachache 43:214, 2003 266. Lipton RB, Stewart WF, Stone AM, et al: Disability in Strategies of Care Study Group. Stratified care vs. step
care strategies for migraine: the Disability in Strategies of Care (DISC) Study: a
randomized trial. JAMA 284:2599, 2000 267. Slugg PH, Kunkel RS: Complications of methysergide therapy: retroperitoneal fibrosis, mitral
regurgitation, edema, and hemolytic anemia. JAMA 213:297, 1970 268. Cortelli P, Sacquegna T, Albani F, et al: Propranolol plasma levels and relief of migraine. Relationship between
plasma propranolol and 4-hydroxyproranolol concentrations and clinical
effects. Arch Neurol 42:46, 1985 269. Troost BT, Wiles RM: Levetiracetam (LEV) for Treatment of Intractable Headache. Headache 43:588, 2003 270. Solomon GD, Steel JD, Spaccavento LJ: Verapamil prophylaxis of migraine. A double-blind, placebo-controlled
study. JAMA 250:2500, 1983 271. Kahan A, Weber S, Amor B, et al: Nifedipine in the treatment of migraine in patients with Raynaud's
phenomenon. N Engl J Med 308:1102, 1983 272. Meyer JS, Hardenberg J: Clinical effectiveness of calcium entry blockers in prophylactic treatment
of migraine and cluster headaches. Headache 23:266, 1983 273. Lance JW: Headache. Ann Neurol 10:1, 1981 274. Havanka-Kanniainen H, Hokkanen E, Myllyla VV: Efficacy of minodipine in the prophylaxis of migraine. Cephalalgia 5:38, 1985 275. Louis P: A double-blind placebo-controlled prophylactic study of flunarizine (Sibelium) in migraine. Headache 21:235, 1981 276. Amery WK, Caers LI, Aerts TJL: Flunarizine, a calcium entry blocker in migraine prophylaxis. Headache 25:249, 1985 277. Ryan RE RE Sr, Ryan RE Jr, Sudilovsky A: Nadolol: its use in the prophylactic treatment of migraine. Headache 23:26, 1983 278. Andersson K, Vinge E: Adrenoceptor blockers and calcium antagonists in the prophylaxis and treatment
of migraine. Drugs 39:355, 1990 279. Daroff RB, Whitney CM: Treatment of vascular headaches. Headache 26:470, 1986 280. Ziegler DK, Hurwitz A, Hassanein RS, et al: Migraine prophylaxis: a comparison of propranolol and amitriptyline. Arch Neurol 44:486, 1987 281. Diamond S, Solomon GD, Freitag FG, et al: Long-acting propranolol in the prophylaxis of migraine. Headache 27:70, 1987 282. Elkind AH, Friedman AP, Bachman A, et al: Silent retroperitoneal fibrosis associated with methysergide therapy. JAMA 206:1041, 1969 283. Graham J: Cardiac and pulmonary fibrosis during methysergide therapy for headache. Am J Med Sci 254:1, 1967 284. Lipton SA: Prevention of classic migraine headache by digital massage of the superficial
temporal arteries during visual aura. Ann Neurol 19:515, 1986 285. Silberstein S, Mathew N, Saper J, et al: Botulinum toxin type A as a migraine preventative treatment. Headache 40:445, 2000 286. Troost BT, Rosenberg JR, Wiles R: Improvement in intractable headache with repeated botulinum toxin type
A treatment. Neurology 60(Suppl 1):A323, 2003 287. Cui M, Khanijou S, Rubino J, et al: Botulinum toxin A inhibits the inflammatory pain in the rat formalin model [Abstract 246.2]. In: Abstracts of the 30th Annual Meeting of the Society for Neuroscience, November 4–9, 2000. Available at: http://sfn.scholarone.com/itin2000/main.html 288. Ishikawa H, Mitsui Y, Yoshitomi T, et al. Presynaptic effects of botulinum toxin type A on the neuronally evoked
response of albino and pigmented rabbit iris sphincter and dilator muscles. Jpn J Ophthalmol 44:106, 2000 289. Welch MJ, Purkiss JR, Foster KA: Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium
botulinum neurotoxins. Toxicology 38:245, 2000 |