In addition to the common saccular (“berry”) aneurysm, the other morphologic type is fusiform, which most frequently but not exclusively involves the posterior vertebrobasilar system. Fusiform aneurysms are characterized by tortuous dilatation and elongation (dolichoectasia) of an artery and are usually associated with atherosclerosis, hence the synonymous term atherosclerotic aneurysm.
The intracranial arteries have thin media and poorly developed or absent internal elastic lamina; they are particularly prone to develop small outpouchings at arterial branch junctions where developmental defects in the media exist. Histologic study of the saccular aneurysm often demonstrates a defect in the elastic and muscular coat, beginning at the point of origin (neck) from the parent artery. This finding has supported the concept of a developmental origin and created the term congenital aneurysm to designate saccular aneurysms, although these lesions are not present at birth. Factors such as hypertension, degenerative changes in the arterial wall, and heritable connective-tissue disorders as in the Ehlers-Danlos syndrome and neurofibromatosis-1, all play roles in the subsequent development of aneurysms. There is firm evidence of aneurysm disease in first- or second-degree relatives, and there is a distinct association with polycystic kidney disease and aortic coarctation.2
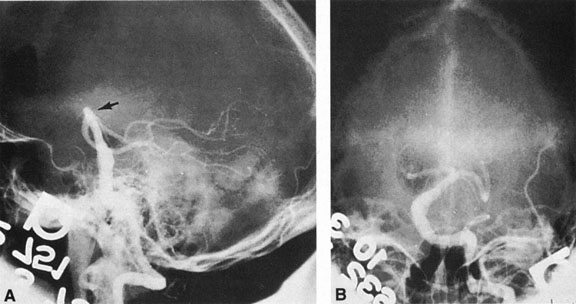
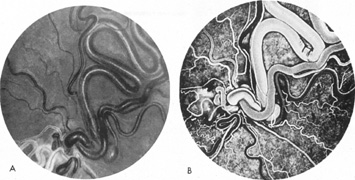
As noted, multiple saccular aneurysms of the carotid or vertebrobasilar system occur 20% to 30% of the time, frequently with identical or mirror sites found in the left-sided and right-sided circulations. Most aneurysms arise from the carotid system on the anterior communicating, middle cerebral, and internal carotid arteries, or they arise in the region of the origins of the posterior communicating arteries (Fig. 1A). Only some 5% of saccular aneurysms arise on the vertebrobasilar system (Fig. 1B).
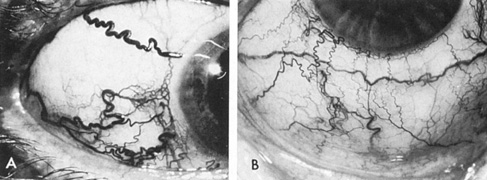
Usually, clinical symptoms are the result of rupture of an aneurysm, resulting in extravasation of blood under arterial pressure into the subarachnoid or intraventricular spaces or into the brain parenchyma, with intracerebral hematoma formation. Subarachnoid and intraventricular blood causes depression of consciousness; intracerebral hematoma and vascular spasm produce focal neurologic signs and even cerebral herniation with compression of the third nerve. The subsequent sudden increase in the intracranial pressure may result in sixth-nerve palsies, in subretinal, intraretinal, or preretinal hemorrhage, and in conjunctival and, rarely, orbital hemorrhage.
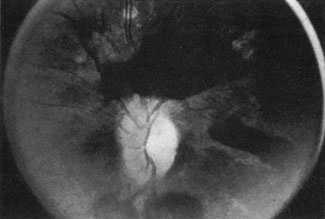
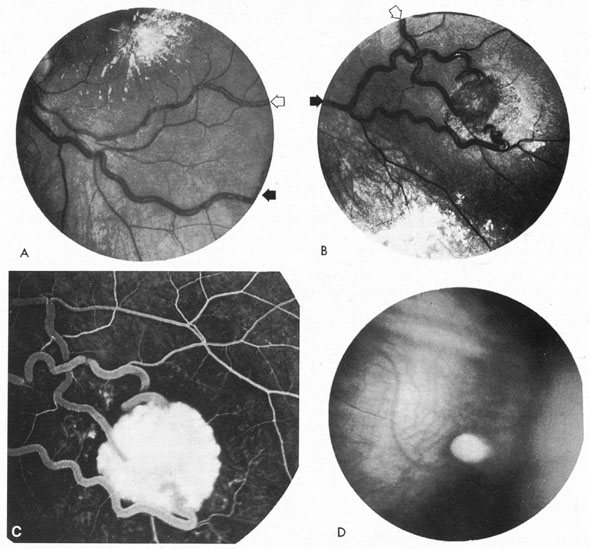
Approximately 100 years ago, Terson recorded the occurrence of vitreous hemorrhage after spontaneous subarachnoid bleeding. Such ocular hemorrhage is believed to result from transmission of intracranial pressure through the subarachnoid communication between the optic nerve sheath and the intracranial cavity, with subsequent nerve sheath dilation and rupture of dural and bridging vessels (Fig. 2). Intraocular hemorrhage is possibly the result of retinal venous hypertension brought on by obstruction of both the central retinal vein and the retinochoroidal anastomoses. Kuhn et al3 have reviewed Terson's syndrome, including the role of pars plana vitrectomy; these authors noted the high incidence of preceding coma, and the efficacy of surgical intervention in visual recovery.
|
Aneurysms, either saccular or fusiform, also cause neurologic deficits by progressive enlargement rather than by rupture. For example, visual loss caused by compression of the optic nerve from fusiform distortion of the supraclinoid carotid or ophthalmic artery, or saccular enlargement of the intracavernous carotid artery that produces chronic ocular motor palsies. Symptoms in patients with aneurysmal compression of the anterior visual pathway may include visual loss that can be acute, gradual, or fluctuating.
SACCULAR ANEURYSMS
Intracavernous Carotid
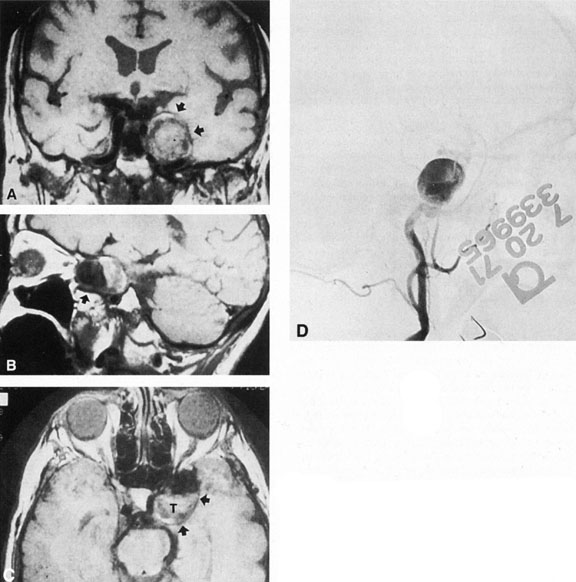
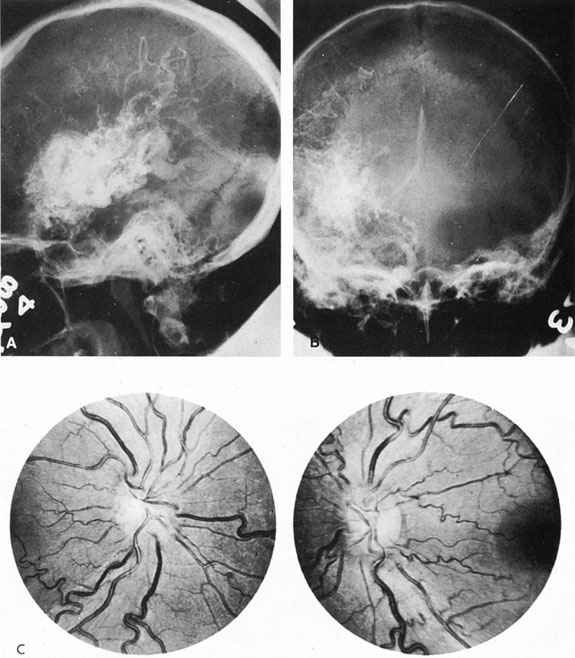
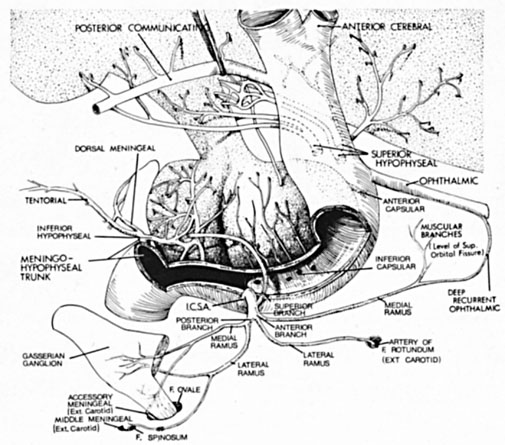
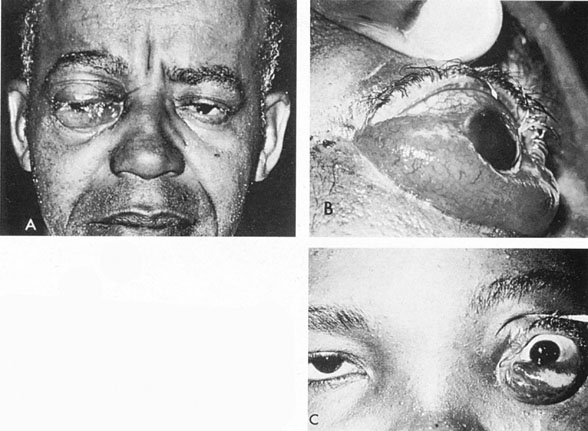
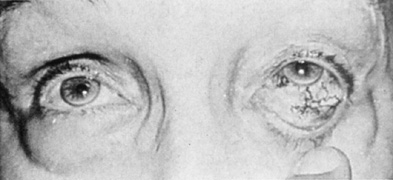
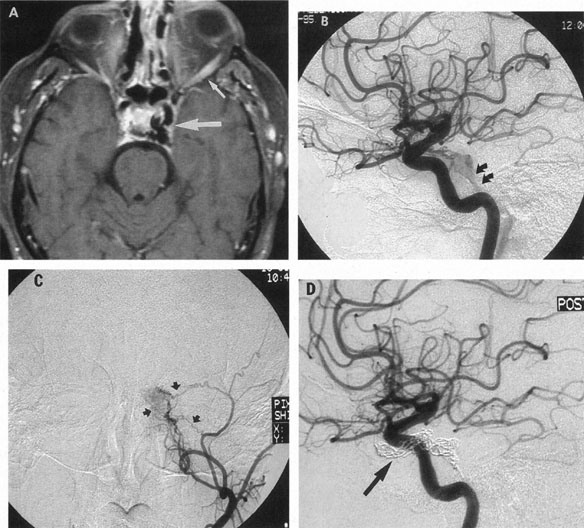
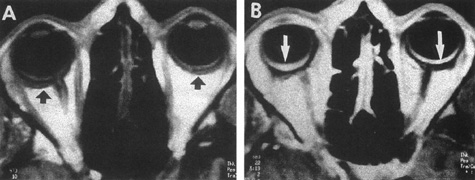
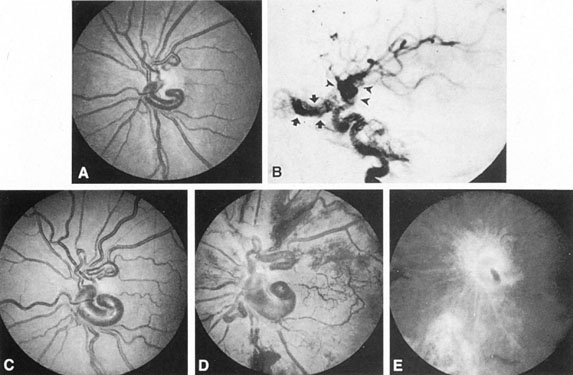
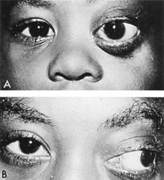
Intracavernous carotid aneurysms constitute only 2% to 3% of all intracranial aneurysms and are unique because of their location. These aneurysms arise from the internal carotid artery as it traverses the cavernous sinus4 (Fig. 3) and therefore produce a specific constellation of ocular and neurologic signs and symptoms. Rupture of such aneurysms, which are almost always saccular, may possibly result in carotid–cavernous sinus fistula, but subarachnoid hemorrhage is rare.5 However, slowly progressive enlargement is the rule, usually occurring within the cavernous sinus, with compression of the third, fourth, and sixth cranial nerves and later involving the first and second divisions of the fifth nerve (see Chapter 12).6 Progressive enlargement of the aneurysm forms a mass in the floor of the middle cranial fossa, compromising motor as well as sensory functions of the trigeminal nerve. Anterior expansion of the aneurysm erodes the anterior clinoid, optic foramen, and superior orbital fissure, eventually producing unilateral visual loss and exophthalmos. Posterior expansion, which occurs later, can erode the petrous portion of the temporal bone, causing ipsilateral facial palsy and, rarely, deafness. The sphenoidal sinus and the nasopharynx may infrequently be involved by inferior expansion and medial extension erodes into the sella and may simulate a pituitary tumor7 or cause bilateral ophthalmoplegia.8 Bilateral saccular intracavernous aneurysms occur uncommonly.9
The onset of signs and symptoms is usually insidious, at times accompanied by pain about the eye and frontal area on the involved side. The pattern of serial involvement of the cranial nerves within the cavernous sinus is usually as follows: sixth, third, fifth, and fourth. Occasionally, palsies evolve simultaneously and the ipsilateral optic nerve may eventually be encroached on by superior expansion of the aneurysm. The pupil is often not dilated maximally, as in the usual acute third-nerve palsy; it may be relatively small (rarely immobile) because of simultaneous involvement of the oculosympathetic fibers.6 Barr and associates4 believed that such aneurysms usually arise as a weakness in the lateral wall of the carotid artery within the cavernous sinus and that the aneurysm tends to expand laterally between the third and fourth nerves superiorly and the sixth nerve inferiorly, finally compressing the nerves on the medial wall of the aneurysmal sac rather than laterally. Late involvement of the fifth nerve is emphasized by the findings that, in the three studied cases, this nerve was not splayed over the lateral aspect of the aneurysm, but that it lay mainly inferior, lateral, and posterior in the region where late expansion of the aneurysm occurs. Although periocular pain has often been emphasized as a prominent symptom, it may not occur until ophthalmoplegia has been present for years.
Intracavernous aneurysms are suspected by the clinical presentation of a chronic cavernous sinus syndrome and are diagnosed by enhanced computed tomography (CT), magnetic resonance imaging (MRI), and arteriography (see Fig. 3). Because of the location and configuration within the cavernous sinus, direct surgical approaches to cavernous carotid aneurysms are hazardous. In recent years intravascular occlusion of the internal carotid by detachable balloon has evolved as a safe and successful procedure, often with relief of pain and improvement in ophthalmoplegia.10 Unfortunately, these balloons are commercially unavailable at the time of this writing.
Carotid–Ophthalmic Artery
The carotid–ophthalmic aneurysm arises from the superior or medial surface of the carotid artery above the cavernous sinus and below the origin of the posterior communicating artery. Such aneurysms have an intimate relation to the anterior clinoid and optic nerve, which tend to cover their point of origin. They are rare in reported series: Pool and Potts11 cited only two examples in 157 cases, and there was a 5.4% incidence in 2,672 patients with single aneurysms in the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage.12 Ferguson and Drake13 reported 32 such aneurysms; Guidetti and LaTorre14 reported 16 cases. A high incidence of concurrent cerebral aneurysms has been reported: 67% in one series14 and 37.5% in another.15
There is a striking correlation between aneurysmal projection and visual impairment. With superior-medial projection, optic nerve compression with monocular visual loss is often present. Eight of 16 patients reported by Guidetti and LaTorre14 had prominent visual symptoms with reduced vision and optic atrophy. Larger aneurysms may involve both optic nerve and chiasm. However, Ferguson and Drake13 reported 32 patients with preoperative visual deficits. While involvement of the anterior visual pathway is the most frequent neuro-ophthalmologic presentation, paralysis of the third cranial nerve was reported in a single case. Although deemed a rare event,13 visual loss occurs in approximately one-third of patients with carotid–ophthalmic aneurysms.15 Aneurysms in this location may traverse the adjacent optic nerve or chiasm by actually splitting it, the mechanism of which is still unknown. Presentation with visual disturbance is again variable.16
Carotid–Supraclinoid
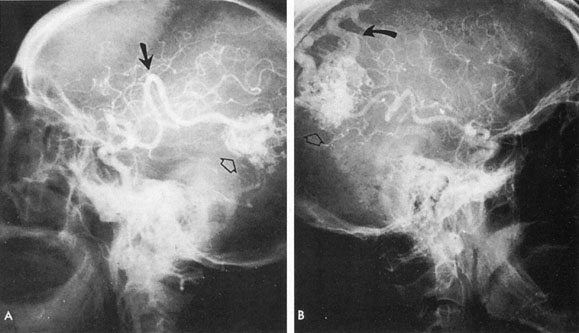
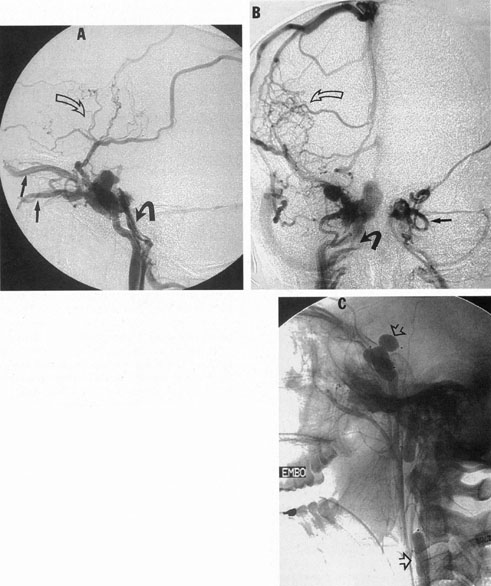
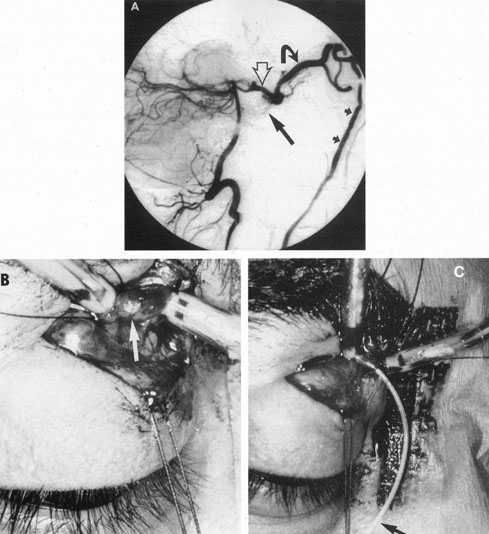
One of the more insidious and potentially treatable causes of progressive visual loss is unruptured aneurysm of the supraclinoid portion of the internal carotid artery. Here, the term supraclinoid seems more a function of size than of specific site or origin, and distinction between ophthalmic and supraclinoidal types is unclear. Of 3,123 giant (larger than 25 mm) intracranial aneurysms in one series,17 93 involved the internal carotid above the cavernous sinus, thus qualifying as supraclinoid. Sixty-five were carotid–ophthalmic, 16 were at the bifurcation of the middle cerebral artery, and 12 were carotid-posterior communicating-anterior choroidal in location. Bilateral carotid–ophthalmic aneurysms were found in 19 cases, with a female predominance and average age of 48 years. The visual system was compressed in all but 6 cases, and 14 aneurysms presented as subarachnoid bleeding. Most of these giant aneurysms occur in women in the fifth and sixth decades of life, and their effects are primarily the result of compression of the optic nerves and chiasm.17 For the most part, involvement of the visual system represents the only neurologic complication. Such aneurysms may rarely rupture, but most commonly they present as insidious visual loss, or they are uncovered during angiography for other sister aneurysms.18 The pattern of visual field loss with supraclinoid aneurysm and its temporal profile tend to differ from that occurring with primary intrasellar or parasellar tumors. Vision is usually affected in a single eye, commonly with nasal field depression, and at times progressing to blindness before the second eye is involved.19 These aneurysms arise below the optic nerve, which is stretched and flattened before subsequent involvement of the chiasm and the opposite nerve.
Most such aneurysms expand upward and forward, becoming located primarily anteriorly (Fig. 4). The optic nerves rise upward form the optic canal and may be inclined at a 45-degree angle such that the chiasm is more superiorly, as well as posteriorly, placed. It may be expected that uniocular ipsilateral visual loss would occur and progress before the contralateral field is involved because of chiasmal compression and before opposite nerve damage ensues. Although rapid visual loss has been reported, a longer duration (even years) is the rule. Rarely, the aneurysm may be more posteriorly placed or the chiasm more anteriorly fixed, resulting in initial involvement of the optic tract.20
Large aneurysms also arise from or involve the origins of the middle or anterior cerebral arteries, and they may precisely mimic a slowly growing suprasellar neoplasm. Similarly, large aneurysms of the supraclinoid carotid can simulate a pituitary tumor,7 including prolactinemia.21 Aneurysms may mimic other masses, and thus underscores the need for MRI or arteriography in the evaluation of some sellar or parasellar syndromes.
Ophthalmic Artery
Intracranial ophthalmic artery aneurysms are rare and in many series are not distinguished from the supraclinoid aneurysms17,19 already discussed. The symptoms are quite similar, consisting of monocular progressive visual loss due to vascular compression from beneath the optic nerve. The pattern of visual loss begins as a unilateral scotoma usually involving fixation and then depression of the nasal and upper field, progressing to blindness. Further expansion involves the lateral aspect of the chiasm, causing temporal field loss in the opposite eye. Rarely, such aneurysms extend to involve the more distal aspects of the ophthalmic artery, producing enlargement of the optic foramen and even erosion into the orbit.19,22 Jain23 reported an unusual presentation of a true saccular aneurysm of the ophthalmic artery with projection into the optic foramen in a patient with monocular papilledema. Again, the intimate relationship of ophthalmic artery origin from the internal carotid, and the size of the aneurysm, may blur distinctions among ophthalmic, carotid–ophthalmic, and supraclinoid.
Intraorbital ophthalmic artery aneurysm was the clinical diagnosis given to the first cases of carotid–cavernous fistula described by Travers24 in 1809. All such previous cases before modern angiography must be suspect, with the majority probably being misdiagnosed carotid–cavernous fistulas or arteriovenous communications involving the orbital circulation. However, cases proved arteriographically do exist. Rubinstein and associates25 reported the case of a 36-year-old man who experienced monocular burning and lacrimation with progressive loss of vision fluctuating over weeks; anigography demonstrated a 7-mm aneurysm arising intraorbitally, 12 mm distal to the origin of the ophthalmic artery. Rarely, direct penetrating trauma may produce aneurysmal dilation of the intraorbital ophthalmic artery.26
Posterior Communicating Artery
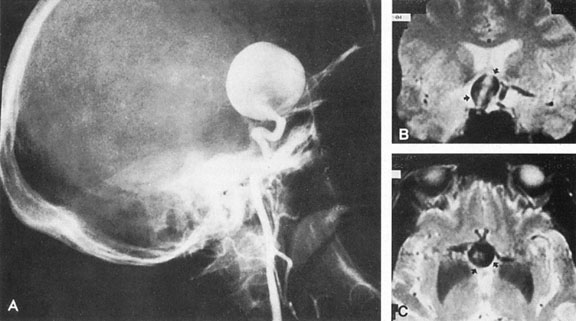
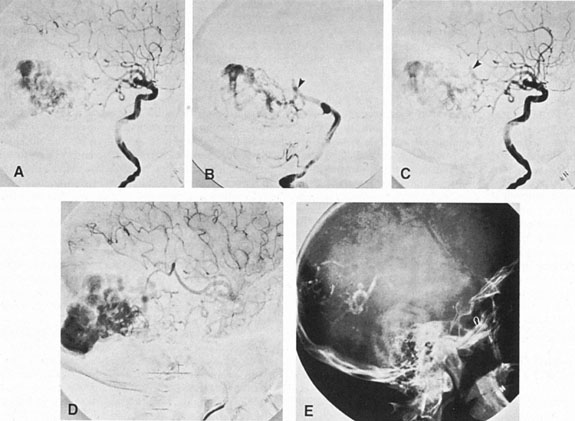
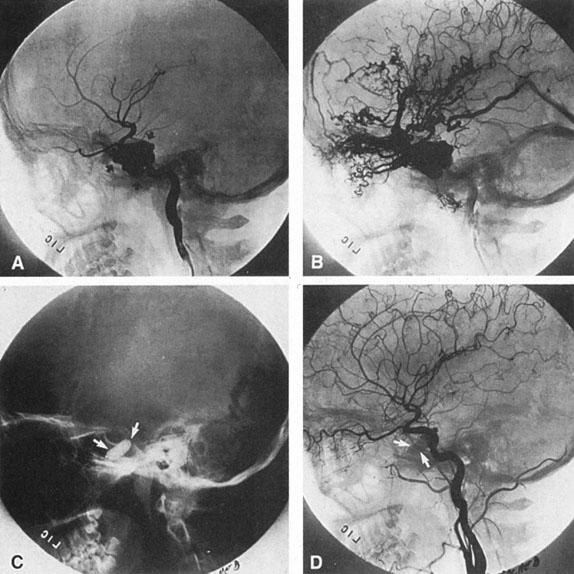
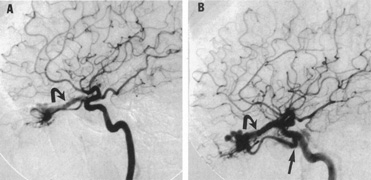
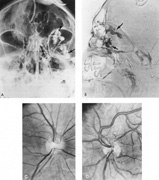
Aneurysms that primarily arise form the carotid system at the origin of the posterior communicating artery are of special interest to neurologists, neurosurgeons, and ophthalmologists because they tend to involve the oculomotor nerve. The classic presentation is sudden onset of severe unilateral frontal headache, ptosis, limitation adduction, depression and elevation of the eye, and dilated and fixed pupil. The cerebrospinal fluid is grossly bloody, and angiography is diagnostic (Fig. 5). Pain in and around the eye in the trigeminal–ophthalmic distribution is a conspicuous symptom, but sensory defects are absent. Clinical and pathologic evidence indicates that impairment of function by a contiguous aneurysm usually occurs in conjunction with hemorrhage into the oculomotor nerve that, along with sudden distortion, can produce referred pain.27
Oculomotor palsy caused by posterior communicating artery aneurysm typically shows maximal involvement of all third nerve functions. Although an individual extraocular muscle may be partially paretic, it is quite uncommon for any single extraocular muscle to be entirely spared (lateral rectus and superior oblique excluded). Relative pupillary sparing and pupillary involvement is of considerable importance in differential diagnosis. The common situation is total pupillary paralysis with ruptured or unruptured posterior communicating aneurysms that involve the third nerve, but important exceptions exist. Of course, in the clinical setting of sudden onset of a painful third-nerve palsy, with severe headache and nuchal rigidity, angiography is indicated whether or not the pupil is involved.
In approximately one-half of patients with ruptured posterior communicating artery aneurysms, third-nerve palsy develops either immediately or within a day. Aneurysms can cause ipsilateral frontal headache and third-nerve palsy by compression before they actually rupture. Approximately 70% of symptomatic but unruptured, aneurysms show a third-nerve palsy, and by far the most common location is at the origin of the posterior communicating artery.12 The incidence of oculomotor palsy with posterior communicating artery aneurysms varies from 34% to 56%.28
After third-nerve palsy, especially when caused by aneurysm or trauma, if recovery does not begin with a few weeks, the phenomenon of misdirection usually occurs (see Chapter 12). Hepler and Cantu29 assessed the ultimate ocular status in 25 patients with third-nerve palsies secondary to aneurysms and found that all patients had some residual abnormality of third nerve function, but it was usually of trivial importance to the patient. Only 5 of 25 patients in this series complained of significant difficulty with diplopia, and 1 paitent had a persistent, complete third-nerve palsy. In another study of patients treated by a direct surgical approach to the posterior communicating artery aneurysm within 10 days of symptomatic onset, a better prognosis existed for recovery of third nerve function than in those operated on after a longer interval.28,30
Middle Cerebral Artery
Aneurysms in the distribution of the middle cerebral artery are common and have a relatively good prognosis even after rupture. These lesions arise at the bifurcation or trifurcation of the middle cerebral artery within the sylvian fissure and usually do not give rise to neuro-ophthalmologic symptomatology until they hemorrhage. Thereafter, blood may dissect into brain parenchyma, producing intracerebral hematomas with resultant contralateral paralysis, sensory loss, and homonymous visual field defect. Rarely, an isolated visual field defect may be the only focal neurologic sign of a bleeding aneurysm located distally on a posterior branch of the middle cerebral artery, with the hematoma having dissected into the visual radiations. Ipsilateral hemicranial, hemifacial, or periorbital pain may herald minor leakage preceding frank subarachnoid hemorrhage.31
Anterior Communicating Artery
The anterior communicating artery is the most common single location of intracranial aneurysm, but these rarely produce focal neurologic signs prior to rupture despite being situated just above the optic nerves. Chan et al32 reported six cases collected over 37 years that caused monocular blindness by rupture of downward pointing aneurysmal sacs. With rupture, bleeding occurs into the optic nerve with symptoms of severe headache and monocular blindness.
Vertebrobasilar System
Between 5% and 15% of all intracranial aneurysms are located in the posterior fossa, but these are often not clinically suspected until rupture. The majority of such aneurysms are at or about the bifurcation of the basilar artery. This location approaches the brainstem exit of the oculomotor nerves, but it is rare that third-nerve palsy occurs prior to aneurysm rupture. Only those aneurysms found just distal to the basilar bifurcation on the proximal posterior cerebral arteries are likely to compromise the oculomotor nerve. When such aneurysms enlarge, they can exert pressure beneath the third ventricle and chiasm simulating the signs of a parasellar tumor, or the aneurysm may invaginate the third ventricle and thus mimic a colloid cyst.33 Saccular vertebrobasilar aneurysms usually present with subarachnoid hemorrhage, without concurrent or premonitory focal neurologic signs and symptoms. Among the 28 patients reported by Hööke et al34 only 8 patients had prerpture symptoms (headache) and 4 had prerupture signs. However, prodromal signs that falsely suggest either vertebrobasilar insufficiency or a posterior fossa mass lesion have been amply documented. We have seen 2 patients who presented with mild subarachnoid hemorrhage from a basilar artery aneurysm but who developed Parinaud's syndrome as the predominant symptomatology, presumably resulting from dorsal midbrain ischemia secondary to vessel spasm. McKinna35 reviewed eye signs in a series of 611 posterior fossa aneurysms and found diplopia, major field defects, retinal or vitreous hemorrhage, or papilledema present in 50%. The double vision (present in 35%) was caused by cranial nerve palsy, skew deviation, defective upward gaze, and bilateral external ophthalmoplegia.
In a series of 50 patients with basilar artery aneurysm, Nijensohn and associates36 found 27 patients with saccular aneurysms and 23 with fusiform basilar dilations. In the former group, symptoms such as episodic diplopia, transient hemiplegia, and paresthesia mimicked the signs and symptoms of vertebrobasilar vascular disease. Various brainstem signs, such as progressive quadriparesis, nystagmus, and multiple cranial nerve involvement, occur with such aneurysms even before rupture. Isolated oculomotor paralysis has been reported with both saccular and fusiform aneurysms of the basilar system.37 Posterior fossa aneurysms can thus mimic mass lesions, with progressive cranial nerve palsies and hydrocephalus.38
Saccular basilar aneurysms tend to present at a younger age (less than 60 years) and are most common in middle-aged women, whereas fusiform ectasia is associated with arteriosclerosis in hypertensive men in the sixth and seventh decades. In one study36 the majority of patients with saccular aneurysms died of rupture, as opposed to those with fusiform basilar artery aneurysms in whom death tended to occur from myocardial infarction. Treatment of such aneurysms presents a formidable technical problem well described elsewhere.39
Posterior Cerebral Artery
Aneurysms of the posterior cerebral artery are rare lesions. Their incidence in the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage was 0.8%.12 Although such aneurysms arise in vessels supplying the major portions of the visual radiations and cortex, Pool and Potts11 remarked that they knew of no instance in which visual symptoms occurred before rupture of the aneurysms. Drake and Amacher40 reported their experience in eight patients, only one of whom had a temporary homonymous field defect after hemorrhage. Rarely, such aneurysms arise from the first portion of the posterior cerebral artery near the junction of the posterior communicating artery; more commonly, they occur at the first major branching of the posterior cerebral artery as it courses around the midbrain. In the former location, ruptured aneurysms are associated with third-nerve palsy and contralateral hemiparesis. These signs might be anticipated because the proximal segment of the posterior cerebral artery is closely related to the cerebral peduncle and third nerve at its emergence between the posterior cerebral artery and superior cerebellar artery.
CHILDHOOD ANEURYSMS
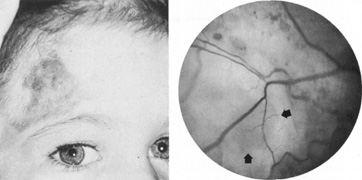
Intracranial aneurysms uncommonly become symptomatic in children, in whom hemorrhage is less frequent and mass effect is more likely. Most intracranial arterial aneurysms seen in children are saccular, and they may be associated with heritable connective tissue disorders, including autosomal dominant polycystic kidney disease, Ehlers-Danlos syndrome type IV, neurofibromatosis-1, and Marfan's syndrome.2 Amacher and Drake41 noted that pediatric aneurysms may reach giant size, may be fusiform in configuration, especially in the vertebrobasilar system, and may be causally related to bacterial endocarditis or other infections. Such mycotic aneurysms are the result of arterial damage from a septic embolus.42 Neurofibromatosis-1 is associated with proliferation of Schwann's cells within arteries, with secondary degenerative changes; in this disorder, there is a predilection for arterial occlusions or aneurysms of the cervical carotid and anterior communicating arteries.43 Fusiform aneurysms of the basilar or carotid arteries, although a common sequel of atheromatous degeneration of the intracranial arteries in adult patients, are rare in children and may be associated with neurofibromatosis-144 or have another pathologic basis.
Patel and Richardson45 found 58 patients younger than 19 years of age (1.9%) among 3,000 cases of ruptured intracranial aneurysms. Of 2,951 patients with cerebral aneurysm in the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage summarized by Locksley,12 only 41 patients showed evidence of aneurysmal rupture by the age of 19 years. Because of their rarity in pediatric patients, aneurysms are infrequently suspected in children with signs of intracranial hemorrhage or mass lesions. Yet, early diagnosis and treatment of aneurysms in children, as in adults, can be lifesaving.
Although there is no doubt that intracranial aneurysm is the most frequent source of subarachnoid hemorrhage in adults, it is a long-established maxim that arteriovenous malformation (AVM) more commonly leads to subarachnoid hemorrhage in children than does aneurysm. Yet, when large series of patients up to 20 years of age with subarachnoid hemorrhage are considered, aneurysm is a more common etiology than AVM. In the 124 young patients with subarachnoid hemorrhage reported by Sedzimir and Robinson46 for example, 50 patients had aneurysms and 33 had AVMs. These authors also summarized 321 patients through 20 years of age, including their own patients and those from three other series, and found 36% with aneurysm and 27% with AVM. Thus, if patients up to age 20 are included, intracranial aneurysms cause subarachnoid bleeding more frequently than do vascular malformations. AVMs predominate if only preadolescent patients with subarachnoid hemorrhage are considered because symptomatic aneurysms are rare in younger groups. There appears to be a biphasic presentation of intracranial aneurysms of the first 2 decades, with the lesions most often becoming symptomatic after age 10 or before the age of 2 years.47 Aneurysms in children rarely become symptomatic between 2 and 10 years of age.
Intracranial aneurysms in children are more common than has previously been recognized owing to a greater awareness, widespread use of noninvasive CT and magnetic resonance angiography (MRA),48 and the relative safety of catheter angiography.49 There are clinical tendencies in children with intracranial aneurysms that differ from those in adults: (i) multiple aneurysms are less common in children; (ii) associated congenital anomalies, especially coarctation of the aorta and polycystic kidneys, are more commonly seen in children with symptomatic aneurysms; (iii) the mortality from initial hemorrhage in children admitted to hospital is less than in adults; and (iv) children tend to recover more quickly and completely from neurologic defects. The usual presentation is intracranial hemorrhage, but ophthalmoplegia, diabetes insipidus, and other signs mimicking tumor have rarely reported with aneurysms.42
TRAUMATIC ANEURYSMS
Secondary (false) aneurysms usually occur after blunt head injury, with or without basilar skull fracture, and also after penetrating injuries to the face or cranium. Traumatic aneurysms of the intracavernous segment of the internal carotid artery come to attention because of recurrent, often massive, epistaxis, with variable involvement of the ocular motor nerves.50 Postsurgical aneurysms are documented after sinus surgery, transsphenodial pituitary resection, and yttrium-90 implantation.51 Despite the large number of transsphenoidal procedures, this complication appears unusual. Paullus and co-workers52 have reported an extraordinary case of progressive bilateral ophthalmoplegia caused by false aneurysm complicated by a carotid–cavernous fistula component. It is to be recalled that, according to microdissections of Rhoton and associates,53 the carotid arteries may approach the midline within the sella to as close as 4 mm, and that adherence to a strictly midline approach seems advisable.
Aneurysms of the extracranial carotid system are distinctly uncommon and may follow trauma or are associated with atherosclerosis or fibromuscular dysplasia.54 Neuro-ophthalmologic signs are rare, including headache or ipsilateral oculosympathetic palsy. Penetrating orbital trauma with false aneurysm of the ophthalmic artery has been noted.26
FUSIFORM ANEURYSMS
Fusiform aneurysms are actually tortuous dilatations and elongations (dolichoectasia) of preexisting arteries, usually associated with advanced but not necessarily caused by atheromatous degeneration, which explains the term atherosclerotic ectasia. The neurologic complications of such dilatations, which can involve either the carotid or vertebrobasilar system, are most often secondary to direct pressure effects rather than to rupture and subarachnoid hemorrhage.
Carotid System
Fusiform carotid aneurysms involve the intracavernous or internal carotid artery. A dilated and tortuous carotid may flatten the optic nerve against the falciform dural fold above the opening of the optic canal. Such compression rarely may be responsible for instances of slowly progressive visual loss with eventual optic atrophy, some instances of which may be mistakenly diagnosed as soft or normal-tension glaucoma.
Hilton and Hoyt55 reported a case of bitemporal hemianopia associated with fusiform dilation of the internal carotid and anterior cerebral arteries. The latter actually prolapsed between the optic nerves. The patient's slowly progressive bitemporal hemianopia and optic atrophy, with normal sella, was not believed to be the result of compression by the dilated vessels but was considered more likely the result of impaired circulation caused by traction on, and obstructions of, the small vessels supplying the anterior aspect of the chiasm. Colapinto et al56 have also described optic nerve compression by fusiform ectasia of internal carotid artery demonstrated by MRI and cerebral angiography (see Chapter 5, Part II).
As previously noted, fusiform aneurysms have been described even in young children and have been associated with connective tissue diseases and neurofibromatosis.2,41,43,44,57 Thus, congenital causes must be suspect in youth, as well as in the situation of spontaneous arterial dissections.
Vertebrobasilar System
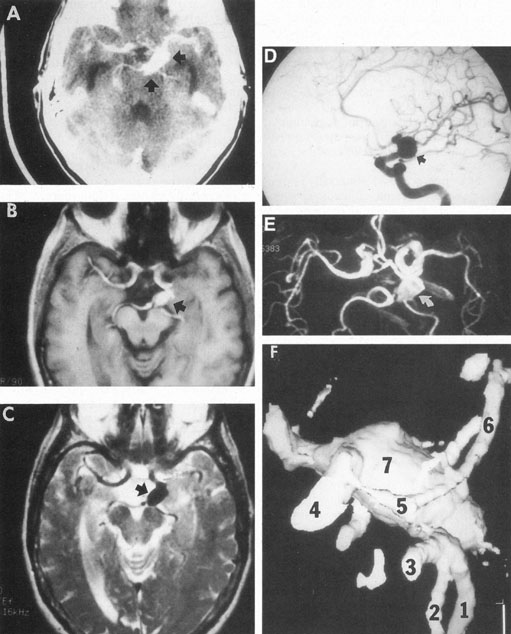
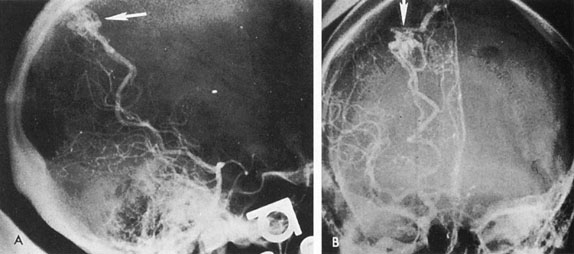
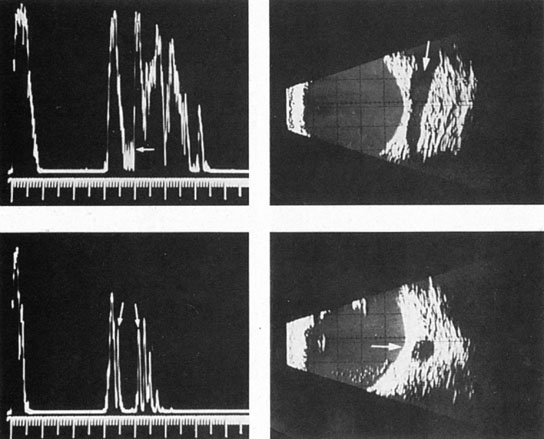
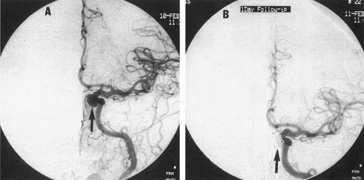
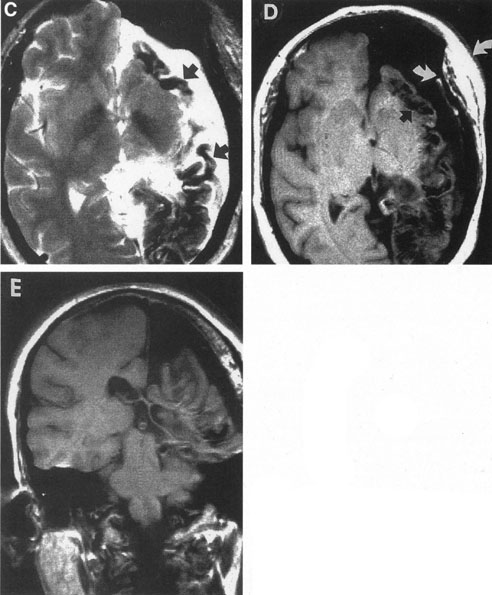
Tortuous or redundant basilar arteries are not uncommon in the older age group. Occasionally, gross dilation or ectasia develops so that the basilar artery acts as a mass in the posterior fossa. This phenomenon produces signs of low-pressure hydrocephalus, cranial nerve palsies, and long tract and sensory signs and may even simulate a cerebellopontine angle tumor or tumor at the foramen magnum.58 It is possible to diagnose such lesions with CT59 or MRI60 but angiography is definitive (Fig. 6). The association of insidious multiple cranial nerve palsies and long tract signs referable to a brainstem level, in an elderly patient with evidence of atherosclerosis, should make fusiform basilar artery dilation a diagnostic consideration.
As opposed to saccular basilar aneurysms, fusiform aneurysms tend to occur in the older age group (older than 60 years) and are found predominately in men.2,36 They are commonly associated with hypertension and atherosclerotic cardiovascular disease, and a notable association with abdominal aortic aneurysms also exists.