ANATOMY
Orbit
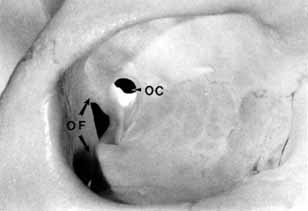
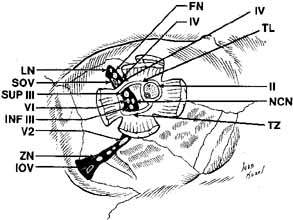
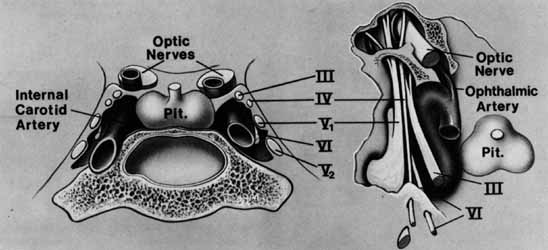
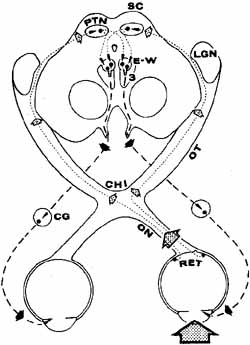
The orbital apex has two major openings, the orbital fissure and the optic canal (Fig. 1). The orbital fissure is divided into a superior and inferior portion by the tendon of Zinn, the inferior insertion for the tendinous anulus of Zinn (Fig. 2).1,4 (The anulus of Zinn is completed by the origins of the rectus muscles and the superior tendon of Lockwood.) Structures emanating through the optic canal (the optic nerve, ophthalmic artery, and orbital sympathetic nerves) as well as some structures coursing through the superior orbital fissure (the superior and inferior branches of the oculomotor nerve (cranial nerve III), the abducens nerve (cranial nerve VI) and the nasociliary branch of the ophthalmic division (cranial nerve V1) of the trigeminal nerve pass through the anulus of Zinn.
|
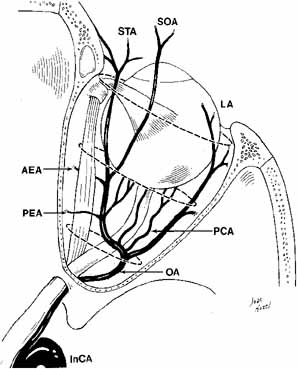
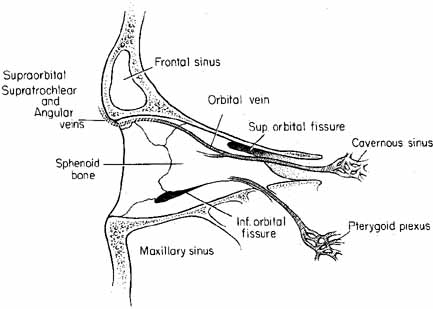
The orbital vascular supply is provided primarily by the ophthalmic artery (Fig. 3), and its branches, the central retinal artery, muscular branches, posterior ciliary arteries, posterior and anterior ethmoidal arteries, and the lacrimal artery. Venous drainage of the orbit is primarily by the superior ophthalmic vein into either the cavernous sinus or the pterygoid plexus (Fig. 4). A variable but generally lesser amount of venous outflow leaves through the inferior orbital vein into the pterygoid plexus. Numerous anastomoses are generally present between the superior and inferior ophthalmic veins.
Cranial Nerve II (Optic Nerve)
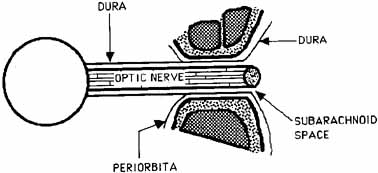
The optic nerve is contained within a dural sheath, which is continuous at the orbital apex with the periosteum of the optic canal. Intracranially the periosteum is continuous with the dura, thus providing access of cerebrospinal fluid to the sub-arachnoid space surrounding the optic nerve (Fig. 5). Elevated cerebrospinal fluid pressure, meningeal inflammation, or air from pneumocephalus can extend into the orbit within the optic sheath (Fig. 6).5
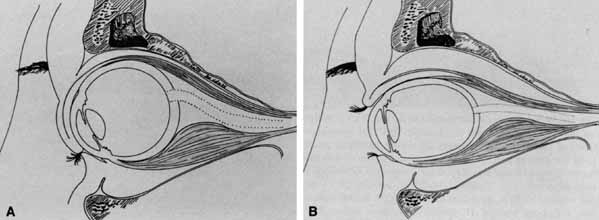
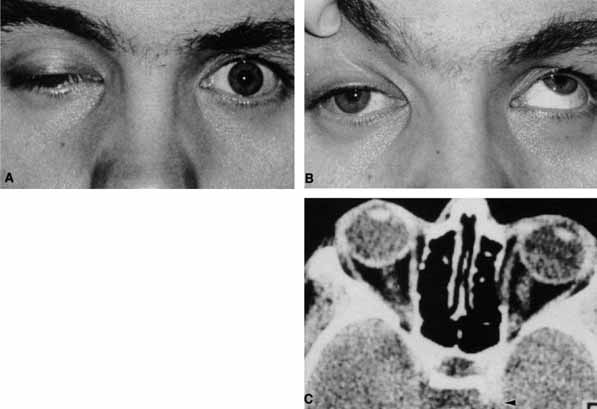
The optic nerve remains vulnerable to compression within the bony confines of the orbit. Compressive optic neuropathy is most likely to occur where the optic nerve is tethered to the bone, that is, at the orbital apex (Fig. 7A). The optic nerve in other areas of the orbit can be displaced markedly without suffering vision loss (Fig. 7B).
Cranial Nerve V (Trigeminal Nerve)
The ophthalmic branch of the trigeminal sensory nerve (cranial nerve V1) arises in the trigeminal ganglion, travels forward within the infralateral wall of the cavernoussinus and divides into its three major branches, the nasociliary, lacrimal, and frontal branches (Fig. 8).
|
The lacrimal and frontal nerves pass above the anulus of Zinn along with the trochlear nerve (cranial nerve IV), the superior ophthalmic vein, and the anastomotic branch of the middle meningeal artery to the lacrimal artery. The frontal branch of cranial nerve V1, provides sensory input from the upper eyelid and forehead, while the lacrimal branch receives its input from the lacrimal gland and the superior temporal eyelid. The lacrimal branch is unique because its terminal branches carry the efferent parasympathetic fibers for lacrimal gland secretion, received from the zygomaticotemporal nerve near the lateral orbital wall.
The nasociliary branch of cranial nerve V1 gives off branches to the globe (long and short posterior ciliary nerves) before terminating as the infratrochlear nerve that provides sensory innervation to the superior medial eyelid. A small, external nasal branch is also given off with the anterior ethmoidal artery that extends under the nasal mucosa to innervate the tip of the nose.
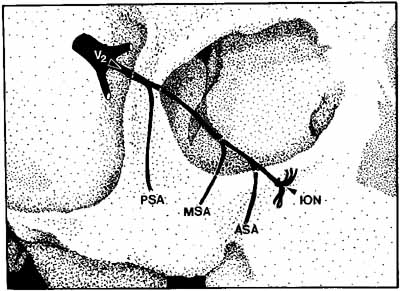
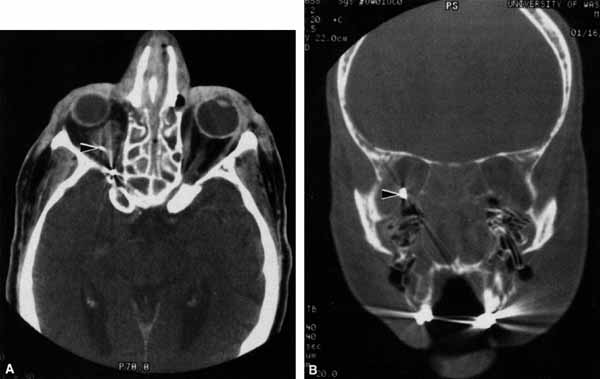
The infraorbital fissure transmits the infraorbital nerve, a branch of the maxillary division (cranial nerve V2) of the trigeminal nerve (see Fig. 2). Branches of the infraorbital nerve rise in the lateral orbit, providing sensation to the temple (zygomaticotemporal nerve) and the lateral malar area (zygomaticofacial nerve) (Fig. 9). The infraorbital nerve continues anteriorly within a bony tunnel until it appears through the maxillary bone to innervate the ipsilateral face to the midline from the lower eyelid to the upper lip. Prior to innervating the face, the infraorbital nerve sends three major descending branches to the upper teeth. The most proximal branch is given off prior to entering the infraorbital fissure (superior posterior alveolar nerve) and innervates the upper molars; the next two branches (middle superior, and anterior superior alveolar nerves) are given off within the infraorbital fissure and canal. These nerves innervate the remainder of the ipsilateral teeth to the midline (Fig. 10). The infraorbital fissure also transmits the inferior orbital vein that drains the inferior orbit into the pterygoid plexus (see Fig. 4).
|
OPTIC NERVE
The tempo and character of vision loss can be important diagnostically. The tempo of visual acuity deterioration varies from optic nerve tumors with characteristically chronic and progressive loss to that of cellulitis or idiopathic orbital inflammation which may be apoplectic. Characteristics of visual change are also important; for instance, gaze-evoked vision loss is most commonly associated with intraconal tumors such as [MT1]optic nerve meningiomas or cavernous hemangiomas.6 This symptom may also occur in patients with orbital fractures, intraconal foreign bodies, or extraconal tumors that compress the central retinal artery.7,8 Transient obscurations of vision, a fleeting blurring of vision lasting seconds, may be associated with papilledema.9 This symptom can be differentiated from amaurosis fugax (complete loss of vision of moderate duration), which is more likely to be secondary to embolic occlusion of the ophthalmic or central retinal artery.10
Every patient with a suspected orbital abnormality deserves a complete eye examination, whether he or she needs it or not.11 Visual acuity with current correction, a manifest refraction, and a cycloplegic refraction are important to detect induced hyperopia from an intraconal tumor. A rough approximation is 3 D of induced hyperopia for each millimeter of globe shortening. Evaluation of afferent visual function should include tests of color vision, relative afferent pupillary defect, and visual field.
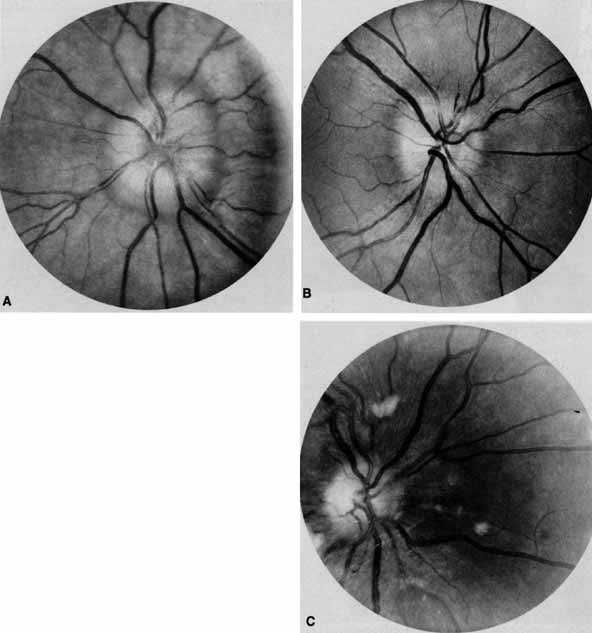
Ophthalmoscopy can be very helpful in orbital diagnosis. Optic disc edema may be caused either by papilledema (increased intracranial pressure) (Fig. 11A), papillitis (anterior optic neuritis) (Fig. 11B), or vasculitis (e.g., lupus erythematosus, sarcoidosis, Wegener's granulomatosis, and giant cell arteritis) (Fig. 11C).12 Papilledema (Fig. 11A) is usually present with near-normal visual function, although some patients may lose vision.9 In contrast, papillitis (Fig. 11B) characteristically leads to reduced visual function, commonly is associated with orbital pain, and is associated with inflammatory cells in the vitreous overlying the disc. Optic disc vasculitis (Fig. 11C) may be present with other findings of vasculitis, and commonly is associated with poor acuity and evidence of vascular occlusion (e.g., cotton-wool spots, hemorrhages). The differential diagnosis of the swollen, erythematous disc is discussed elsewhere in these volumes.
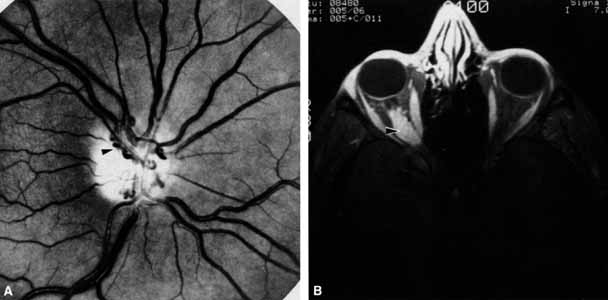
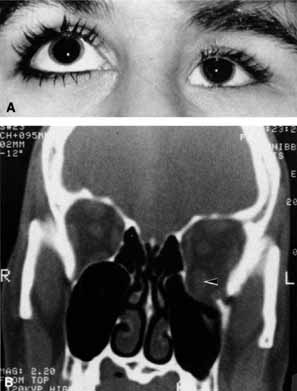
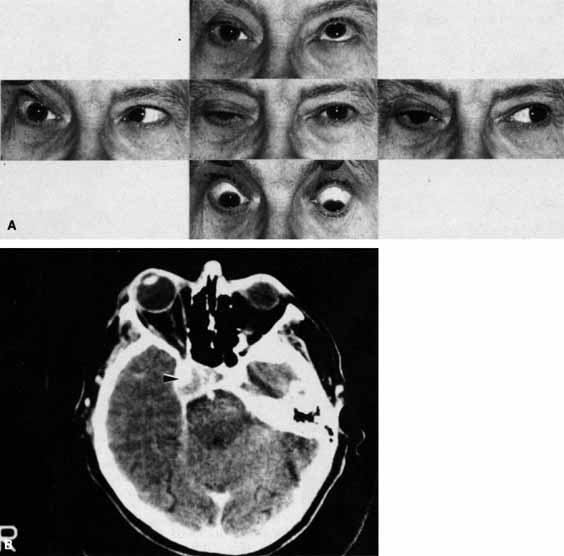
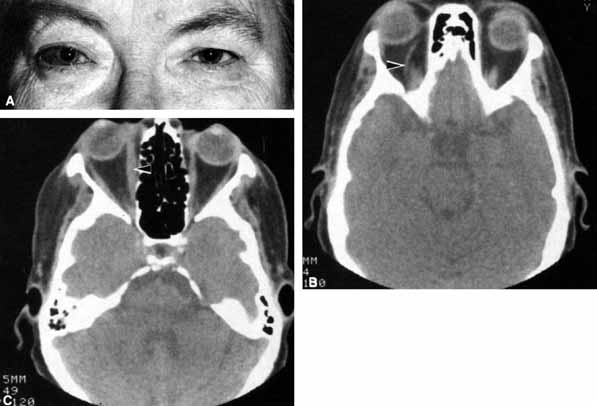
Optociliary shunts (Fig. 12A) develop as a result of long-term obstruction of the central retinal vein. This may occur secondary to optic nerve meningiomas,13 optic nerve gliomas,14,15 optic nerve meningoceles,16 central retinal vein occlusions,17 optic nerve sarcoidosis,18 craniosynostosis,19 optic nerve arachnoid cysts,20 optic disc drusen,21 and papilledema.15 Congenital optociliary shunts, which are exceedingly rare, have not been associated with optic neuropathy or orbital disease.22,23 The presence of optic nerve pallor, poor acuity and optociliary shunts generally implies an optic nerve meningioma in the absence of other funduscopic findings (Fig. 12B).13
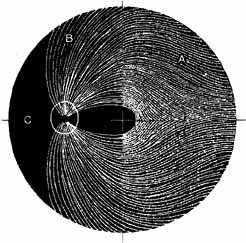
Visual field testing can also be helpful in diagnosis of orbital processes. Optic disc–associated visual field changes are usually altitudinal because of the structure of the horizontal raphé (Fig. 13). This visual field defect is typically a result of anterior ischemic optic neuropathy (AION).24 Compressive lesions of the orbit or retrobulbar inflammation are more likely to produce a central or cecocentral scotoma. However, one must be aware that there is a significant degree of overlap between various types of optic nerve lesions and visual field abnormalities.25 In these cases, neuroimaging may be a useful adjunct in differentiating between different neuro-ophthalmic and orbital processes.26
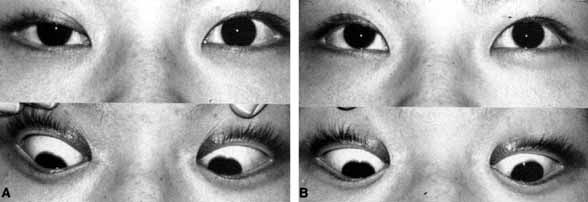
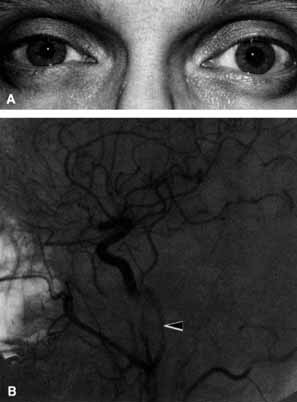
The relationship of visual function to orbital findings often helps in the differential diagnosis. For instance, patients with cavernous hemangiomas may have significant proptosis but retain normal, best-corrected visual function (see Fig. 7B), but patients with intrinsic optic nerve meningiomas may demonstrate minimal proptosis with significant vision loss (see Fig. 12B).
Trigeminal Sensation
Historically, the progression of numbness may be helpful in diagnosis of orbital disease. For instance, adenoid cystic carcinoma of the lacrimal gland27,28 and some melanomas29 have a predilection for perineural invasion. The presence of a lacrimal gland mass, which is followed by numbness in the distribution of the lacrimal (see Fig. 2), zy-gomaticotemporal, or zygomaticofacial nerves (Fig. 9), can indicate perineural extension of an adenoid cystic carcinoma.
Corneal sensation should always be tested prior to anesthetizing the cornea for applanation tonometry. Corneal sensation can be roughly quantified using an anesthesiometer; however, asymmetry of corneal sensation may be the most important finding in unilateral orbital disease. A cotton wisp, pulled from a cotton-tip applicator, is lightly drawn from the conjunctiva across the limbus onto the cornea. The blink should occur with corneal but not conjunctival touch. Sensory testing of cranial nerves V1 and V2 with the cotton wisp can be performed at the same time. The cutaneous distribution of the zygomaticofacial and zygomatico-temporal branches should not be ignored (see Fig. 9). The upper teeth should also be tested by tapping with the cotton-tip applicator stick, comparing right to left sides and the front teeth to the molars. Blowout fractures that involve the infraorbital nerve commonly result in numbness of the upper teeth because the anterior and middle superior alveolar nerves arise from the infraorbital nerve within the infraorbital canal (see Fig. 10). However, because the posterior superior alveolar nerve arises from the infraorbital nerve before it enters the infraorbital canal, if the molars are also numb, one must consider the possibility of an associated basilar skull fracture.
Pain is a helpful diagnostic symptom. There are five types of pain associated with the eye and orbit11,19,30:
- Pain with eye movement
- Pain with palpation (tenderness)
- Ocular pain
- Orbital pain
- Referred pain
Structures that are pain-sensitive within the orbit are the optic nerve sheath, the extraocular muscle sheaths, and the intermuscular septae. Stretching of any of these structures, if they are inflamed, will produce pain on ocular movement. Optic neuritis, myositis, sinusitis with secondary myositis, or cellulitis may result in pain with eye movement. Most of these entities may also lead to constant orbital pain that increases with eye movement.
Tenderness is characteristic of anterior orbital disease that either distends or inflames periostium or Tenon's capsule. Orbital masses that are commonly tender are leaking dermoid cysts, mucoceles, dacryoadenitis, and orbital pseudotumor involving Tenon's capsule (scleritis).
Examples of ocular pain secondary to ocular disease include photophobia caused by keratitis, the aching of uveitis, and the pain of angle-closure glaucoma. These diagnoses are generally evident on complete eye examination and are discussed in their respective chapters.
Orbital pain caused by orbital disease is constant, deep-seated behind the eye, moderate to severe in intensity, and usually unilateral, but not migratory. There is usually little doubt in either the patient's or the examiner's mind about the presence of true orbital pain, as compared with referred pain (see further on in text). Orbital diseases that commonly produce pain include adenoid cystic carcinoma of the lacrimal gland, cellulitis, idiopathic orbital inflammation, and acute orbital hemorrhage.
Referred pain (Table 1) is the most common cause of orbital pain (see case 6). Tension headache is probably the most common cause of referred orbital pain. Tension headaches are generally localized to the periorbital region, often to the brows, and are more diffusely localized than true orbital pain.11 The pain is commonly bilateral or may migrate from side to side. The pain is usually mild, rarely awaking the patient from sleep, and is controlled by mild analgesics.
TABLE 1. Referred Orbital Pain
| Tension headache |
| Occipital neuralgia |
| Nasopharyngeal carcinoma |
| Intracranial or intracavernous aneurysms |
| Cavernous arteriovenous malformation |
| Cavernous sinus inflammation (e.g., Tolosa-Hunt syndrome) |
Fleeting, stabbing, sharp pains so commonly mentioned by patients are usually referred pains. In the face of a normal ophthalmic and neurologic examination these fleeting pains are unassociated with a pathologic process.30
Greater occipital neuralgia may also lead to referred pain. The trigeminal nerve nucleus, which has its afferent input in the pons, has a spinal extension to the level of C1–2. An afferent input from the greater occipital nerve at C1 or C2 can stimulate the spinal nucleus of the trigeminal nerve by ephaptic transmission resulting in referred orbital pain.31
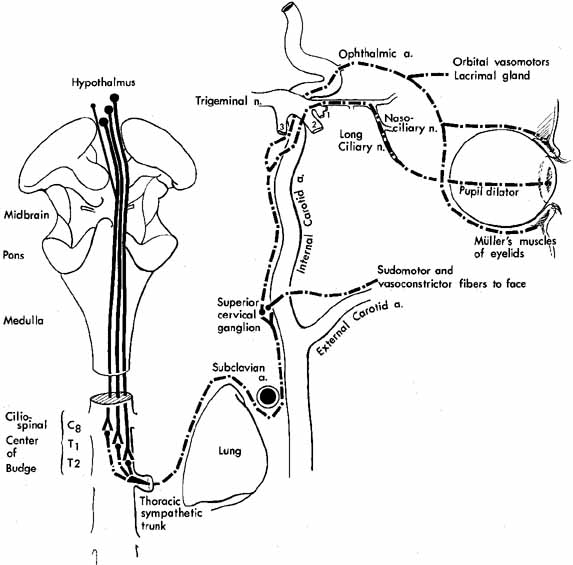
Branches of the ophthalmic division of the trigeminal nerve also contribute to the afferent arc of the oculocardiac reflex. Sudden pressure on the eyeball, acute elevation of intraocular pressure or stretching the extraocular muscles can cause symptoms ranging from nausea and syncope to potentially life-threatening bradycardia. Afferent fibers from cranial nerve V1 pass through polysynaptic pathways in the reticular formation before synapsing with visceral motor nuclei of the vagus nerve, which activate efferent parasympathetic fibers that slow the heart rate.32 Clinical scenarios where an ophthalmologist may encounter this include manipulation of extraocular muscles during strabismus surgery, acute angle-closure glaucoma, herniation of rectus muscles after blowout fractures,33 and acute retrobulbar hemorrhages. In addition to administering intravenous atropine to pharmacologically increase the heart rate, the acute management of these patients may require emergent decompression of the orbit by releasing the lateral canthal tendon, immediate reduction of orbital fractures and reposition of herniated orbital contents,33 and peripheral iridotomy for acute angle-closure glaucoma.