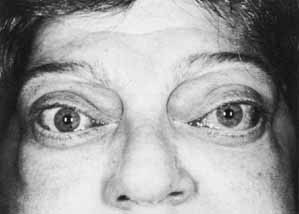
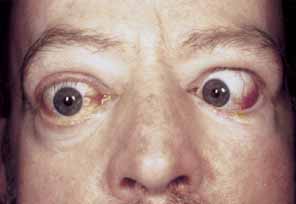
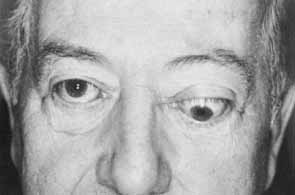
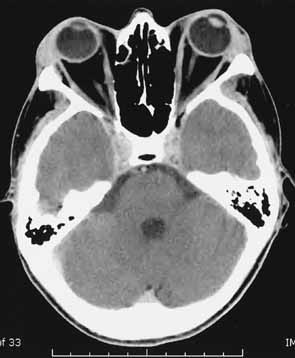
1. Parry CH: Collections from the Unpublished Medical Writings, vol. 11. London: Underwoods, 1825 2. Graves RJ: Newly observed affection of the thyroid gland in females. London Med Surg J 7:516–517, 1935 3. von Basedow K: Exophthalmos durch hypertrophic des zellgewebes in der augenhohle. Wochens Ges Heilkd 13:197, 1840 4. Elgood C (ed):Medicine in Persia. New York: Hoeber, 1934 5. Fries PD: Thyroid dysfunction: Managing the ocular complications of Graves' disease. Geriatrics 47:58–60, 1992 6. Salvi M, Zhang Z-G, Haegert D, et al: Patients with endocrine ophthalmopathy not associated with overt thyroid
disease have multiple thyroid immunological abnormalities. J Clin Endocrinol Metab 70:89–94, 1990 7. Bartley GB, Fatourechi V, Kadrmas EF, et al: The incidence of Graves' ophthalmopathy in Olmsted County, Minnesota. Am J Ophthalmol 120:511–517, 1995 8. Bahn RS: Clinical review 157. Pathophysiology of Graves' ophthalmopathy: The
cycle of disease. J Clin Endocrinol Metab 88:1939–1946, 2003 9. Burch HB, Wartofsky L: Graves' ophthalmopathy: Current concepts regarding pathogenesis and
management. Endocr Rev 14:747–793, 1993 10. Kriss JP: Pathogenesis and treatment of Graves' ophthalmopathy. Thyroid Today 7:1–9, 1984 11. Forbes G, Gorman CA, Brennan MD, et al: Ophthalmopathy of Graves' disease: Computerized volume measurements
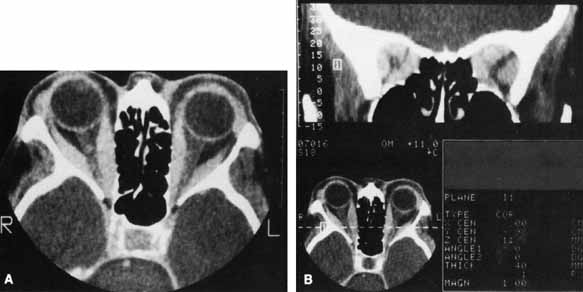
of the orbital fat and muscle. Am J Neuroradiol 7:651–656, 1986 12. Chang TC, Huang KM, Chang TJ, et al: Correlation of orbital computed tomography and antibodies in patients with
hyperthyroid Graves' disease. Clin Endocrinol (Oxf) 32:551–558, 1990 13. Marcocci C, Bartalena L, Bogazzi F, et al: Studies on the occurrence of ophthalmopathy in Graves' disease. Acta Endocrinol (Copenh) 120:473–478, 1989 14. Kalman K, Leovey A, Kelenhegyi C, et al: Euthyroid infiltrative ophthalmopathy: Clinical-immunological characteristics. Acta Med Hung 46:101–108, 1989 15. Prummel MF, Wiersinga WM, Mourits MP, et al: Effect of abnormal thyroid function on the severity of Graves' ophthalmopathy. Arch Intern Med 150:1098–1101, 1990 16. Bartley GB, Fatourechi V, Kadrmas EF, et al: The chronology of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol 121:426–434, 1996 17. Tamai H, Nakagawa T, Ohsako N, et al: Changes in thyroid functions in patients with euthyroid Graves' disease. J Clin Endocrinol Metab 50:108–112, 1980 18. Gorman CA: The presentation and management of endocrine ophthalmopathy. Clin Endocrinol Metab 7:67–96, 1978 19. Tellez M, Cooper J, Edmonds C: Graves' ophthalmopathy in relation to cigarette smoking and ethnic
origin. Clin Endocrinol (Oxf) 36:291–294, 1992 20. Christensen SB, Ericsson UB, Janzon L, et al: Influence of cigarette smoking on goiter formation, thyroglobulin, and
thyroid hormone levels in women. J Clin Endocrinol Metab 58:615–618, 1984 21. Ericsson U-B, Lindgärde F: Effects of cigarette smoking on thyroid function and the prevalence of
goitre, thyrotoxicosis and autoimmune thyroiditis. J Intern Med 229:67–71, 1991 22. Kahaly G, Moncayo R, Stover C, et al: Relationship of eye muscle antibodies with HLA phenotypes and thyroid-stimulating
immunoglobulins in endocrine orbitopathy. Res Exp Med 191:137–144, 1991 23. Sridama V, Hara Y, Fauchet R, et al: HLA immunogenic heterogeneity in black American patients with Graves' disease. Arch Intern Med 147:229–231, 1987 24. Kawa A, Nakamura S, Nakazawa M, et al: HLA-B35 and B5 in Japanese patients with Graves' disease. Acta Endocrinol (Copenh) 86:754–757, 1977 25. Hancock SL, Cox RS, McDougall IR: Thyroid diseases after treatment of Hodgkin's disease. N Engl J Med 325:599–605, 1991 26. Adams DD, Purves HD: Abnormal responses in the assay of thyrotropin. Proc Univ Otago Med Schove 34:11–12, 1956 27. Kriss JP, Pleshakov V, Chien JR: Isolation and identification of the long-acting thyroid stimulator
and its relation to hyperthyroidism and circumscribed pretibial myxedema. J Clin Endocrinol Metab 24:1005–1028, 1964 28. Gunji K, Kubota S, Stolarski C, et al: A 63 kDa skeletal muscle protein associated with eye muscle inflammation
in Graves' disease is identified as the calcium binding protein
calsequestrin. Autoimmunity 29:1–9, 1999 29. Noh JY, Hamada N, Inoue Y, et al: Thyroid-stimulating antibody is related to Graves' ophthalmopathy, but
thyrotropin-binding inhibitor immunoglobulin is related
to hyperthyroidism in patients with Graves' disease. Thyroid 10:809–813, 2000 30. Gerding MN, van der Meer JWC, Broenink M, et al: Association of thyrotrophin receptor antibodies with the clinical features
of Graves' ophthalmopathy. Clin Endocrinol 52:267–271, 2000 31. Morris JCIII , Hay ID, Nelson RE, et al: Clinical utility of thyrotropin-receptor antibody assays: Comparison
of radioreceptor and bioassay methods. Mayo Clin Proc 63:707–717, 1988 32. Gorman CA, Garrity JA, Fatourechi V, et al: The aftermath of orbital radiotherapy for Graves' ophthalmopathy. Ophthalmology 109:2100–2107, 2002 33. Weightman DR, Perros P, Sherif IH, et al: Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmology. Autoimmunity 16:251–257, 1993 34. Heufelder AE: Pathogenesis of Graves' ophthalmopathy: Recent controversies and progress. Eur J Endocrinol 132:532–541, 1995 35. De Carli M, D'Elios MM, Mariotti S, et al: Cytolytic T cells with Th1-like cytokine profile predominate in
retroorbital lymphocytic infiltrates of Graves' ophthalmopathy. J Clin Endocrinol Metab 77:1120–1124, 1993 36. Pritchard J, Horst N, Cruikshank W, et al: Igs from patients with Graves' disease induce the expression of T
cell chemoattractants in their fibroblasts. J Immunol 168:942–950, 2002 37. Sorisky A, Pardasani D, Gagnon A, et al: Evidence of adipocyte differentiation in human orbital fibroblasts in primary
culture. J Clin Endocrinol Metab 81:3428–3431, 1996 38. Jakobiec FA, Bilyk JR, Font RL: Orbit. In: Spencer WH (ed): Ophthalmic Pathology: An Atlas and Textbook, vol 4, 4th ed. Philadelphia WB Saunders, 1996:2438–2933 39. Tallstedt L, Norberg R: Immunohistochemical staining of normal and Graves' extraocular muscle. Invest Ophthalmol Vis Sci 29:175–184, 1988 40. Trokel SL, Jakobiec FA: Correlation of CT scanning and pathological features of ophthalmic Graves' disease. Ophthalmology 88:553–564, 1981 41. Campbell RJ: Immunology of Graves' ophthalmopathy: Retrobulbar histology and histochemistry. Acta Endocrinol 121(suppl 2):9–16, 1989 42. Werner SC: Classification of the eye changes of Graves' disease (Editorial). J Clin Endocrinol Metab 29:982–984, 1969 43. Werner SC: Modification of the classification of the eye changes of Graves' disease: Recommendations
of the Ad Hoc Committee of the American Thyroid
Association (Letter). J Clin Endocrinol Metab 44:203–204, 1977 44. Bartalena L, Marcocci C, Bogazzi F, et al: A new ophthalmopathy index for quantitation of eye changes of Graves' disease. Acta Endocrinol 121(suppl 2):190–192, 1989 45. Gorman CA: The measurement of change in Graves' ophthalmopathy. Thyroid 8:539–543, 1998 46. Wiersinga WM, Smit T, van der Gaag R, et al: Clinical presentation of Graves' ophthalmopathy. Ophthalmic Res 21:73–82, 1989 47. Migliori ME, Gladstone GJ: Determination of the normal range of exophthalmometric values for black
and white adults. Am J Ophthalmol 98:438–442, 1984 48. Amino N, Yuasa T, Yabu Y, et al: Exophthalmos in autoimmune thyroid disease. J Clin Endocrinol Metab 51:1232–1234, 1980 49. Anderson RL, Tweeten JP, Patrinely JR, et al: Dysthyroid optic neuropathy without extraocular muscle involvement. Ophthalmic Surg 20:568–574, 1989 50. Hamed LM, Lessner AM: Fixation duress in the pathogenesis of upper eyelid retraction in thyroid
orbitopathy. A prospective study. Ophthalmology 101:1608–1613, 1994 51. Lemke BN: Anatomic considerations in upper eyelid retraction. Ophthal Plast Reconstr Surg 7:158–166, 1991 52. Bartley GB, Fatourechi V, Kadrmas EF, et al: Long-term follow-up of Graves ophthalmopathy in an incidence
cohort. Ophthalmology 103:958–962, 1996 53. Coleman DJ, Jack RL, Franzen LA, et al: High resolution B-scan ultrasonography of the orbit. Eye changes
in Graves' disease. Arch Ophthalmol 88:465–471, 1972 54. Fells P, Kousoulides L, Pappa A, et al: Extraocular muscle problems in thyroid eye disease. Eye 8:497–505, 1994 55. Gupta MK, Beham JP, Sheeler LR, et al: Effect of 131Iodine therapy on the course of Graves' ophthalmopathy: A quantitative
analysis of extraocular muscle volumes using orbital magnetic resonance
imaging. Thyroid 11:959–965, 2001 56. Nugent RA, Belkin RI, Niegel JM, et al: Graves orbitopathy: Correlation of CT and clinical findings. Radiology 177:675–682, 1990 57. Prummel MF, Bakker A, Wiersinga WM, et al: Multi-center study on the characteristics and treatment strategies
of patients with Graves' orbitopathy: The first European group
on Graves' orbitopathy experience. Eur J Endocrinol 148:491–495, 2003 58. Weinstein GS, Dresner SC, Slamovits TL, et al: Acute and subacute orbital myositis. Am J Ophthalmol 96:209–217, 1983 59. Koornneef L: Eyelid and orbital fascial attachments and their clinical significance. Eye 2:130–134, 1988 60. Spierer A, Eisenstein Z: The role of increased intraocular pressure on upgaze in the assessment
of Graves' ophthalmopathy. Ophthalmology 98:1491–1494, 1991 61. Kikkawa DO, Cruz RCJr , Christian WK, et al: Botulinum A toxin injection for restrictive myopathy of thyroid-related
orbitopathy: Effects on intraocular pressure. Am J Ophthalmol 135:427–431, 2003 62. Ohtsuka K: Intraocular pressure and proptosis in 95 patients with Graves' ophthalmopathy. Am J Ophthalmol 124:570–572, 1997 63. Khalil HA, de Keizer RJ, Kijlstra A: Analysis of tear proteins in Graves' ophthalmopathy by high performance
liquid chromatography. Am J Ophthalmol 106:186–190, 1988 64. Kadrmas EF, Bartley GB: Superior limbic keratoconjunctivitis. A prognostic sign for severe Graves' ophthalmopathy. Ophthalmology 102:1472–1475, 1995 65. Trobe JD: Optic nerve involvement in dysthyroidism. Ophthalmology 88:488–492, 1981 66. Day RM, Carroll FD: Optic nerve involvement associated with thyroid dysfunction. Arch Ophthalmol 67:289–297, 1962 67. Henderson JW: Optic neuropathy of exophthalmic goiter (Graves' disease). Arch Ophthalmol 59:471–480, 1958 68. Neigel JM, Rootman J, Belkin RI, et al: Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology 95:1515–1521, 1988 69. Soares-Welch CV, Fatourechi V, Bartley GB, et al: Optic neuropathy of Graves' disease: Results of transantral orbital
decompression and long-term follow-up in 215 patients. Am J Ophthalmol 136:433–441, 2003 70. Gorman CA: Temporal relationship between onset of Graves' ophthalmopathy and
diagnosis of thyrotoxicosis. Mayo Clin Proc 58:515–519, 1983 71. Spencer CA: Clinical utility and cost-effectiveness of sensitive thyrotropin
assays in ambulatory and hospitalized patients. Mayo Clin Proc 63:1214–1222, 1988 72. Yeh SD: Symptoms, diagnosis and treatment of thyroid disease. Compr Ther 17:12–18, 1991 73. Shammas HJF, Minckler DS, Ogden C: Ultrasound in early thyroid orbitopathy. Arch Ophthalmol 98:277–279, 1980 74. Forrester JV, Sutherland GR, McDougall IR: Dysthyroid ophthalmopathy: Orbital evaluation with B-scan ultrasonography. J Clin Endocrinol Metab 45:221–224, 1977 75. Rothfus WE, Curtin HD: Extraocular muscle enlargement: A CT review. Radiology 151:677–681, 1984 76. Shah KJ, Dasher BG, Brooks B: Computed tomography of Graves' ophthalmopathy. Diagnosis, management
and posttherapeutic evaluation. Clin Imaging 13:58–61, 1989 77. Ott M, Breiter N, Albrecht CF, et al: Can contrast enhanced MRI predict the response of Graves' ophthalmopathy
to orbital radiotherapy? Br J Radiol 75:514–517, 2002 78. Prummel MF, Gerding MN, Zonneveld FW, et al: The usefulness of quantitative orbital magnetic resonance imaging in Graves' ophthalmopathy. Clin Endocrinol 54:205–209, 2001 79. Just M, Kahaly G, Higer HP, et al: Graves' ophthalmopathy: Role of MR imaging in radiation therapy. Radiology 179:187–190, 1991 80. Patrinely JR, Osborn AG, Anderson RL, et al: Computed tomographic features of nonthyroid extraocular muscle enlargement. Ophthalmology 96:1038–1047, 1989 81. Weetman AP: Overview of Graves' autoimmune disease. In Dutton JJ, Haik BG (eds): Thyroid Eye Disease: Diagnosis and Treatment. New York: Marcel Dekker, 2002:97–106 82. Schaaf L, Rominger-Seyrich D, Grossmann E, et al: Reduction by orbital radiation of cumulative doses of gluco-corticosteroids
in patients with Graves'-Basedow ophthalmopathy. Dev Ophthalmol 20:155–158, 1989 83. Beck RW, DiLoreto DA: Treatment of Graves' ophthalmopathy (Letter). N Engl J Med 322:1088–1089, 1990 84. Solomon B, Glinoer D, Lagasse R, et al: Current trends in the management of Graves' disease. J Clin Endocrinol Metab 70:1518–1524, 1990 85. Dunn JT: Thyroglobulin, hormone synthesis and thyroid disease. Eur J Endocrinol 132:603–604, 1995 86. Weetman AP, McGregor AM, Hall R: Evidence for an effect of antithyroid drugs on the natural history of Graves' disease. Clin Endocrinol (Oxf) 21:163–172, 1984 87. Orgiazzi J: Management of Graves' hyperthyroidism. Endocrinol Metab Clin North Am 16:365–389, 1987 88. Meyer-Gessner M, Benker G, Lederbogen S, et al: Antithyroid drug-induced agranulocytosis: Clinical experience with
ten patients treated at one institution and review of the literature. J Endocrinol Invest 17:29–36, 1994 89. Scott GR, Forfar JC, Toft AD: Graves' disease and atrial fibrillation: The case for even higher
doses of therapeutic iodine-131. Br Med J 289:399–400, 1984 90. Wade JS, Goodall P, Deane L, et al: The course of partial parathyroid insufficiency after thyroidectomy. Br J Surg 52:497–503, 1965 91. Hales IB, Rundle FF: Ocular changes in Graves' disease. A long-term follow-up
study. Q J Med 29:113–126, 1960 92. Perros P, Crombie AL, Kendall-Taylor P: Natural history of thyroid associated ophthalmopathy. Clin Endocrinol 42:45–50, 1995 93. Hamilton HE, Schultz RO, DeGowin EL: The endocrine eye lesion in hyperthyroidism. Its incidence and course in 165 patients
treated for thyrotoxicosis with iodine-131. Arch Intern Med 105:675–685, 1960 94. Calissendorff BM, Söderström M, Alveryd A: Ophthalmopathy and hyperthyroidism—a comparison between patients
receiving different antithyroid treatments. Acta Ophthalmol 64:698–703, 1986 95. Sridama V, DeGroot LJ: Treatment of Graves' disease and the course of ophthalmopathy. Am J Med 87:70–73, 1989 96. Bartley GB, Fatourechi V, Kadrmas EF, et al: The treatment of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol 121:200–206, 1996 97. Tallstedt L, Lundell G, Torring O, et al: Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism. The
Thyroid Study Group. N Engl J Med 326:1733–1738, 1992 98. Bartalena L, Marcocci C, Bogazzi F, et al: Use of corticosteroids to prevent progression of Graves' ophthalmopathy
after radioiodine therapy for hyperthyroidism. N Engl J Med 321:1349–1352, 1989 99. Manso PG, Furlanetto RP, Wolosker AMB, et al: Prospective and controlled study of ophthalmopathy after radioiodine therapy
for Graves' hyperthyroidism. Thyroid 8:49–52, 1998 100. Tallstedt L, Lundell G, Blomgren H, et al: Does early administration of thyroxine reduce the development of Graves' ophthalmopathy
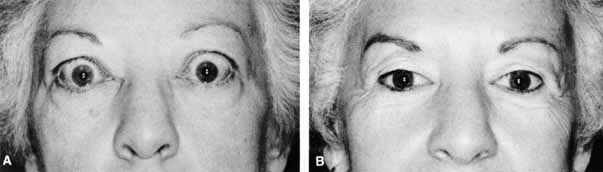
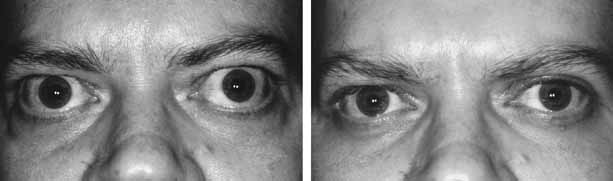
after radioiodine treatment? Eur J Endocrinol 130:494–497, 1994 101. Shine B, Fells P, Edwards OM, et al: Association between Graves' ophthalmopathy and smoking. Lancet 335:1261–1263, 1990 102. Sergott RC, Felberg NT, Savino PJ, et al: Graves' ophthalmopathy: Immunologic parameters related to corticosteroid
therapy. Invest Ophthalmol Vis Sci 20:173–182, 1981 103. Sisson JC, Vanderburg JA: Lymphocyte-retrobulbar fibroblast interaction: Mechanisms by which
stimulation occurs and inhibition of stimulation. Invest Ophthalmol Vis Sci 111:15–20, 1972 104. Wiersinga WM, Smit T, Schuster-Uittenhoeve ALJ, et al: Therapeutic outcome of prednisone medication and of orbital irradiation
in patients with Graves' ophthalmopathy. Ophthalmologica 197:75–84, 1988 105. Prummel MF, Mourits MP, Berghout A, et al: Prednisone and cyclosporine in the treatment of severe Graves' ophthalmopathy. N Engl J Med 321:1353–1359, 1989 106. Zgliczynski S, Jastrzebska H, Górowski T, et al: Treatment of severe Graves' ophthalmopathy: a three-stage management: Corticotherapy, retrobulbar irradiation, and transantral decompression. Acta Endocrinol 121(suppl 2):169–178, 1989 107. Apers RC, Oosterhuis JA, Bierlaagh JJ: Indications and results of prednisone treatment in thyroid ophthalmopathy. Ophthalmologica 173:163–167, 1976 108. Ivy HK: Medical approach to ophthalmopathy of Graves' disease. Mayo Clin Proc 47:980–985, 1972 109. Ohtsuka K, Sato A, Kawaguchi S, et al: Effect of high-dose intravenous steroid pulse therapy followed by 3-month
oral steroid therapy for Graves' ophthalmopathy. Jpn J Ophthalmol 46:563–567, 2002 110. Ohtsuka K, Sato A, Kawaguchi S, et al: Effect of steroid pulse therapy with and without orbital radiotherapy on
Graves' ophthalmopathy. Am J Ophthalmol 135:285–290, 2003 111. Macchia PE, Bagattini M, Lupoli G, et al: High-dose intravenous corticosteroid therapy for Graves' ophthalmopathy. J Endocrinol Invest 24:152–158, 2001 112. Kauppinen-Makelin R, Karma A, Leinonen E, et al: High-dose intravenous methylprednisolone pulse therapy versus oral
prednisone for thyroid-associated ophthalmopathy. Acta Ophthalmol Scand 80:316–321, 2002 113. Shipley ME, Bacon PA, Berry H, et al: Pulsed methylprednisolone in active early rheumatoid disease: A dose-ranging
study. Br J Rheumatol 27:211–214, 1988 114. Chikanza C, Fernandes L: Arrhythmia after pulse methylprednisolone therapy. Br J Rheumatol 30:392–393, 1991 115. Schonwald S: Methylprednisolone anaphylaxis. Am J Emerg Med 17:583–585, 1999 116. Utech C, Wulle KG, Panitz N, et al: Treatment of Graves' ophthalmopathy with new immunosuppressive agents. Dev Ophthalmol 20:94–99, 1989 117. Weetman AP, McGregor AM, Ludgate M, et al: Cyclosporin improves Graves' ophthalmopathy. Lancet 2:486–489, 1983 118. Bigos ST, Nisula BC, Daniels GH, et al: Cyclophosphamide in the management of advanced Graves' ophthalmopathy. Ann Intern Med 90:921–923, 1979 119. Perros P, Weightman DR, Crombie AL, et al: Azathioprine in the treatment of thyroid-associated ophthalmopathy. Acta Endocrinol 122:8–12, 1990 120. Kahaly G, Lieb W, Muller-Forell W, et al: Ciamexone in endocrine orbitopathy. A randomized, double-blind, placebo-controlled
study. Acta Endocrinol 122:13–21, 1990 121. Kahaly G, Pitz S, Muller-Forell W, et al: Randomized trial of intravenous immunoglobulins versus prednisolone in
Graves' ophthalmopathy. Clin Exp Immunol 106:197–202, 1996 122. Wiersinga WM, Prummel MF: Graves' ophthalmopathy: A rational approach to treatment. Trends Endocr Met 13:280–287, 2002 123. Kolodziej-Maciejewska H, Peterski Z: Positive effect of bromocriptine treatment in Graves disease orbitopathy. Exp Clin Endocrinol 86:241–242, 1985 124. Balazs C, Kiss E, Farid NR: Inhibitory effect of pentoxifylline on HLA-DR expression and gylcosaminoglycan
synthesis of retrobulbar fibroblasts induced by interferon
gamma. Acta Microbiol Immunol Hung 44:173–179, 1997 125. Bartalena L, Pinchera A, Marcocci C: Management of Graves' ophthalmopathy: Reality and perspectives. Endocr Rev 21:168–199, 2000 126. Bouzas EA, Karadimas P, Mastorakos G, et al: Antioxidant agents in the treatment of Graves' ophthalmopathy. Am J Ophthalmol 129:618–622, 2000 127. Antonelli A, Saracino A, Alberti B, et al: High-dose intravenous immunoglobulin treatment in Graves' ophthalmopathy. Acta Endocrinol 126:13–23, 1992 128. Kelly WF, Longson D, Smithard D, et al: An evaluation of plasma exchange for Graves' ophthalmopathy. Clin Endocrinol 18:485–493, 1983 129. Glinoer D, Schrooyen M: The treatment of severe Graves' ophthalmopathy with plasma exchange
and immunosuppression. Acta Endocrinol (Copenh) 121:149–153, 1989 130. Zielinski CC, Weissel M, Muller C, et al: Long-term follow-up of patients with Graves' orbitopathy
treated by plasmapheresis and immunosuppression. Dev Ophthalmol 20:130–138, 1989 131. Fakhri O: The use of low voltage electric therapy in the treatment of Graves' ophthalmopathy. Int Ophthalmol 15:201–203, 1991 132. Donaldson SS, Bagshaw MA, Kriss JP: Supervoltage orbital radiotherapy for Graves' ophthalmopathy. J Clin Endocrinol Metab 37:276–285, 1973 133. Maalouf T, George JL, Angioi-Duprez K, et al: Effects of orbital radiotherapy on extraocular muscles in Graves' ophthalmopathy. Orbit 15:25–32, 1996 134. Mourits MP, van Kempen-Harteveld ML, García MBG, et al: Radiotherapy for Graves' orbitopathy: Randomised placebo-controlled
study. Lancet 355:1505–1509, 2000 135. Bartalena L, Marcocci C, Chiovato L, et al: Orbital cobalt irradiation combined with systemic corticosteroids for Graves' ophthalmopathy: Comparison with systemic corticosteroids alone. J Clin Endocrinol Metab 56:1139–1144, 1983 136. Hurbli T, Char DH, Harris J, et al: Radiation therapy for thyroid eye diseases. Am J Ophthalmol 99:633–637, 1985 137. Teng CS, Crombie AL, Hall R, et al: An evaluation of supervoltage orbital irradiation for Graves' ophthalmopathy. Clin Endocrinol 13:545–551, 1980 138. van Ruyven RLJ, van den Bosch WA, Mulder PGH, et al: The effect of retrobulbar irradiation on exophthalmos, ductions and soft
tissue signs in Graves' ophthalmopathy: A retrospective analysis
of 90 cases. Eye 14:761–764, 2000 139. Gorman CA, Garrity JA, Fatourechi V, et al: A prospective, randomized, double-blind, placebo-controlled
study of orbital radiotherapy for Graves' ophthalmopathy. Ophthalmology 108:1523–1534, 2001 140. Wilson WB, Prochoda M: Radiotherapy for thyroid orbitopathy. Effects on extraocular muscle balance. Arch Ophthalmol 113:1420–1425, 1995 141. Prummel MF, Mourits MP, Blank L, et al: Randomised double-blind trial of prednisone versus radiotherapy
in Graves' ophthalmopathy. Lancet 342:949–954, 1993 142. Kahaly GJ, Rösler H-P, Pitz S, et al: Low-versus high-dose radiotherapy for Graves' ophthalmopathy: A
randomized, single blind trial. J Clin Endocrinol Metab 85:102–108, 2000 143. Nakahara H, Noguchi S, Murakami N, et al: Graves ophthalmopathy: MR evaluation of 10 Gy versus 24 Gy irradiation
combined with systemic corticosteroids. Radiology 196:857–862, 1995 144. Kriss JP, Petersen IA, Donaldson SS, et al: Supervoltage orbital radiotherapy for progressive Graves' ophthalmopathy: Results
of a twenty-year experience. Acta Endocrinol 121:154–159, 1989 145. Leone CR: The management of ophthalmic Graves' disease. Ophthalmology 91:770–779, 1984 146. Kinyoun JL, Kalina RE, Brower SA, et al: Radiation retinopathy after orbital irradiation for Graves' ophthalmopathy. Arch Ophthalmol 102:1473–1476, 1984 147. Brown GC, Shields JA, Sanborn G, et al: Radiation retinopathy. Ophthalmology 89:1494–1501, 1982 148. Nygaard B, Specht L: Transitory blindness after retrobulbar irradiation of Graves' ophthalmopathy. Lancet 351:725–726, 1998 149. Snijders-Keilholz A, De Keizer RJW, Goslings BM, et al: Probable risk of tumour induction after retro-orbital irradiation
for Graves' ophthalmopathy. Radiother Oncol 38:69–71, 1996 150. Marcocci C, Bartalena L, Bogazzi F, et al: Orbital radiotherapy combined with high dose systemic glucocorticoids for
Graves' ophthalmopathy is more effective than radiotherapy alone: Results
of a prospective randomized study. J Endocrinol Invest 14:853–860, 1991 151. Wiersinga WM, Prummel MF: An evidence-based approach to the treatment of Graves' ophthalmopathy. Endocrinol Metab Clin North Am 29:297–319, 2000 152. Panzo GJ, Tomsak RL: A retrospective review of 26 cases of dysthyroid optic neuropathy. Am J Ophthalmol 96:190–194, 1983 153. Guy JR, Fagien S, Donovan JP, et al: Methylprednisolone pulse therapy in severe dysthyroid optic neuropathy. Ophthalmology 96:1048–1053, 1989 154. Kazim M, Trokel S, Moore S: Treatment of acute Graves' orbitopathy. Ophthalmology 98:1443–1448, 1991 155. Dollinger J: Die Druckentlastung der augenhöhle durch entfernung der äuberen
orbitalwand bei hochgradigem exophthalmus (morbus Basedowii) und
konsekutiver Hornhauterkrankung. Deutsche Medizin Wochenschr 41:1888–1890, 1911 156. Long JC, Ellis GD: Temporal decompression of the orbit for thyroid exophthalmos. Am J Ophthalmol 62:1089–1098, 1966 157. Berke RN: A modified Krönlein operation. Trans Am Ophthalmol Soc 51:193–231, 1953 158. Naffziger HC: Progressive exophthalmos following thyroidectomy: Its pathology and treatment. Ann Surg 1994:582–584, 1931 159. Hirsch O: Surgical decompression of malignant exophthalmos. Arch Otolaryngol 51:325–334, 1950 160. Ogura JH, Lucente FE: Surgical results of orbital decompression for malignant exophthalmos. Laryngoscope 84:637–644, 1974 161. Kennerdell JS, Maroon JC: An orbital decompression for severe dysthyroid exophthalmos. Ophthalmology 89:467–472, 1982 162. Walsh TE, Ogura JH: Transantral orbital decompression for malignant exophthalmos. Laryngoscope 67:544–568, 1957 163. Anderson RL, Linberg JV: Transorbital approach to decompression in Graves' disease. Arch Ophthalmol 99:120–124, 1981 164. Carter KD, Frueh BR, Hessburg TP, et al: Long-term efficacy of orbital decompression for compressive optic
neuropathy of Graves' eye disease. Ophthalmology 98:1435–1442, 1991 165. Long JA, Baylis HI: Hypoglobus following orbital decompression for dysthyroid ophthalmopathy. Ophthal Plast Reconstr Surg 6:185–189, 1990 166. Scherer H: Orbital decompression surgery. Dev Ophthalmol 20:169–172, 1989 167. McCord CD: Current trends in orbital decompression. Ophthalmology 92:21–33, 1985 168. Hurwitz J, Rosenstock T: Management of inadequate transantral orbital decompression with extended
lateral orbitotomy. Can J Ophthalmol 18:194–196, 1983 169. Wulc AE, Popp JC, Bartlett SP: Lateral wall advancement in orbital decompression. Ophthalmology 97:1358–1369, 1990 170. Leone CR, Piest KL, Newman RJ: Medial and lateral wall decompression for thyroid ophthalmopathy. Am J Ophthalmol 108:160–166, 1989 171. Goldberg RA, Kim AJ, Kerivan KM: The lacrimal keyhole, orbital door jamb, and basin of the inferior orbital
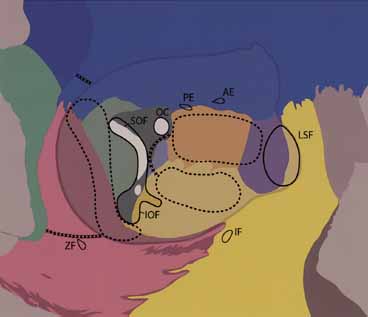
fissure. Arch Ophthalmol 116:1618–1624, 1998 172. Tessier P: Expansion chirurgicale de l'orbite. Ann Chir Plast 14:207–214, 1969 173. Lyons CJ, Rootman J: Orbital decompression for disfiguring exophthalmos in thyroid orbitopathy. Ophthalmology 101:223–230, 1994 174. ünal M, Ileri F, Konuk O, et al: Balanced orbital decompression combined with fat removal in Graves' ophthalmopathy. Ophthal Plast Reconstr Surg 19:112–118, 2003 175. Paridaens D, Hans K, van Buitenen S, et al: The incidence of diplopia following coronal and translid orbital decompression
in Graves' orbitopathy. Eye 12:800–805, 1998 176. Nunery WR, Nunery CW, Martin RT, et al: The risk of diplopia following orbital floor and medial wall decompression
in subtypes of ophthalmic Graves' disease. Ophthal Plast Reconstr Surg 13:153–160, 1997 177. Schaefer SD, Soliemanzadeh P, Della Rocca DA, et al: Endoscopic and transconjunctival orbital decompression for thyroid-related
orbital apex compression. Laryngoscope 113:508–513, 2003 178. Hutchison BM, Kyle PM: Long-term visual outcome following orbital decompression for dysthyroid
eye disease. Eye 9:578–581, 1995 179. Garrity JA, Fatourechi V, Bergstralh EJ, et al: Results of transantral orbital decompression in 428 patients with severe
Graves' ophthalmopathy. Am J Ophthalmol 116:533–547, 1993 180. Tallstedt L, Papatziamos G, Lundblad L, et al: Results of transantral orbital decompression in patients with thyroid-associated
ophthalmopathy. Acta Ophthalmol Scand 78:206–210, 2000 181. Seiff SR, Tovilla JL, Carter SR, et al: Modified orbital decompression for dysthyroid orbitopathy. Ophthal Plast Reconstr Surg 16:62–66, 2000 182. Goldberg RA, Shorr N, Cohen MS: The medial orbital strut in the prevention of postdecompression dystopia
in dysthyroid ophthalmopathy. Ophthal Plast Reconstr Surg 8:32–34, 1992 183. Mourits MP, Koornneef L, Wiersinga WM, et al: Orbital decompression for Graves' ophthalmopathy by inferomedial, by
inferomedial plus lateral, and by coronal approach. Ophthalmology 97:636–641, 1990 184. Linberg JV, Anderson RL: Transorbital decompression. Indications and results. Arch Ophthalmol 99:113–119, 1981 185. White WA, White WL, Shapiro PE: Combined endoscopic medial and inferior orbital decompression with transcutaneous
lateral orbital decompression in Graves' orbitopathy. Ophthalmology 110:1827–1832, 2003 186. Kacker A, Kazim M, Murphy M, et al: “Balanced” orbital decompression for severe Graves' orbitopathy: Technique
with treatment algorithm. Otolaryngol Head Neck Surg 128:228–235, 2003 187. Shepard KG, Levin PS, Terris DJ: Balanced orbital decompression for Graves' ophthalmopathy. Laryngoscope 108:1648–1653, 1998 188. Goldberg RA, Weinberg DA, Shorr N, et al: Maximal, three-wall, orbital decompression through a coronal approach. Ophthalmic Surg Lasers 28:832–843, 1997 189. Gorman CA, DeSanto LW, MacCarty CS, et al: Optic neuropathy of Graves' disease. Treatment by transantral or transfrontal
orbital decompression. N Engl J Med 290:70–75, 1974 190. Colvard DM, Waller RR, Neault RW, et al: Nasolacrimal duct obstruction following transantral-ethmoidal orbital
decompression. Ophthalmic Surg 10:25–28, 1979 191. Crawford BA: Thyroid optic neuropathy: To decompress or not? Aust J Ophthalmol 1:6–11, 1973 192. Olivari N: Transpalpebral decompression of endocrine ophthalmopathy (Graves' disease) by
removal of intraorbital fat: Experience with 147 operations
over 5 years. Plast Reconstr Surg 87:627–641, 1991 193. Adenis JP, Robert PY, Lasudry JGH, et al: Treatment of proptosis with fat removal orbital decompression in Graves' ophthalmopathy. Eur J Ophthalmol 8:246–252, 1998 194. Olivari N: Thyroid-associated orbitopathy: Transpalpebral decompression by
removal of intraorbital fat. Experience with 1362 orbits in 697 patients
over 13 years. Exp Clin Endocrinol Diabetes 107:S208–S211, 1999 195. Ferreira MC, Tuma PJr , Costa MP, et al: Surgical treatment of endocrine exophthalmos by removal of orbital fat: Clinical
experience. Rev Hosp Clín Fac Med 57:217–222, 2002 196. Dunn WJ, Major MC, Arnold AC, et al: Botulinum toxin for the treatment of dysthyroid ocular myopathy. Ophthalmology 93:470–475, 1986 197. Lyons CJ, Vickers SF, Lee JP: Botulinum toxin therapy in dysthyroid strabismus. Eye 4:538–542, 1990 198. Biglan AW, Burnstine RA, Rogers GL, et al: Management of strabismus with botulinum A toxin. Ophthalmology 96:935–943, 1989 199. Coats DK, Paysse EA, Plager DA, et al: Early strabismus surgery for thyroid ophthalmopathy. Ophthalmology 106:324–329, 1999 200. Boergen KP: Surgical repair of motility in Graves' orbitopathy. Dev Ophthalmol 20:159–168, 1989 201. Gardner TA, Kennerdell JS: Treatment of dysthyroid myopathy with adjustable suture recession. Ophthalmic Surg 21:519–521, 1990 202. Lueder GT, Scott WE, Kutschke PJ, et al: Long-term results of adjustable suture surgery for strabismus secondary
to thyroid ophthalmopathy. Ophthalmology 99:993–997, 1992 203. Scott WE, Thalacker JA: Diagnosis and treatment of thyroid myopathy. Ophthalmology 88:493–498, 1981 204. Nguyen VT, Park DJJ, Levin L, et al: Correlation of restricted extraocular muscle motility in surgical management
of strabismus in Graves' ophthalmopathy. Ophthalmology 109:384–388, 2002 205. Gerding MN, Terwee CB, Dekker FW, et al: Quality of life in patients with Graves' ophthalmopathy is markedly
decreased: Measurement by the medical outcomes study instrument. Thyroid 7:885–889, 1997 |