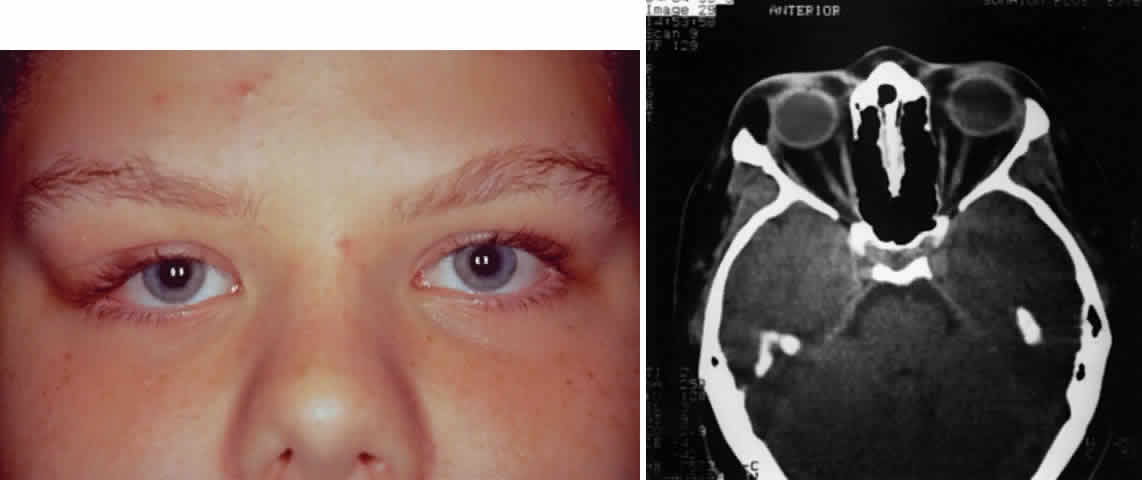
The main lacrimal gland is a bilobed, flat, oval mass of approximately 78 g. It occupies the space between the globe and orbital roof in the superior temporal quadrant of the orbit.7 The lobulated, grayish pink surface of the lacrimal gland allows one to easily distinguish this gland from surrounding orbital tissue. In addition, a thin pseudocapsule covers the gland, which assists in surgically defining a tissue plane between some lacrimal gland neoplasms and normal orbital tissue.8,9 The inferior surface of the lacrimal gland contours to the shape of the globe and is therefore slightly concave. The superior convex surface fills a shallow depression in the anterior lateral aspect of the frontal bone called the fossa glandulae lacrimalis, or lacrimal gland fossa. The lacrimal gland fossa may be seen on plain radiographs as a smooth line arching over the superior temporal orbital rim.
The lacrimal gland is divided into two lobes by the lateral edge of the levator palpebrae aponeurosis. The orbital lobe, which is situated in a posterosuperior position, is two to three times larger than the palpebral lobe, which is situated in an anteroinferior position. The posterior edge of the orbital lobe is in the approximate coronal plane of the posterior pole of the globe. The anterior margin of the orbital portion rests on the superior surface of the levator palpebrae aponeurosis and is covered by orbital septum, orbicularis oculi muscle, and skin. The medial edge of the orbital lobe is situated within the preaponeurotic fat pocket anteriorly and approximates the lateral margin of the superior rectus muscle posteriorly. The lateral edge of the orbital lobe extends inferiorly toward the superior margin of the lateral rectus muscle.10 The palpebral lobe is located under the levator aponeurosis and just above the superior lateral conjunctival fornix. It is this portion of the lacrimal gland that is often visible when the upper eyelid is everted.
The lacrimal gland is supported by four fascial structures, including Soemmering's ligaments, Whitnall's ligament, the inferior ligament of Schwalbe, and the lateral horn of the levator palpebrae aponeurosis.11 The majority of fibers that make up Whitnall's ligament pass under and through the orbital lobe to insert on the orbital roof, providing the greatest support for the lacrimal gland.12 The superior surface of the lacrimal gland is weakly adherent to the periosteum of the lacrimal gland fossa by fine trabecular ligaments known as Soemmering's ligaments. The lateral edge of the levator aponeurosis provides additional support as it divides the gland into two lobes. The inferior ligament of Schwalbe is a small band of fascia, associated with the lacrimal artery and nerve, that passes under the posterior lip of the lacrimal gland. The anterior margin of this ligament and the lateral margin of the levator aponeurosis define an oval opening referred to as the lacrimal foramen.11 It is through this foramen that ductules of the orbital lobe pass into the palpebral lobe. These ductules intertwine with ductules of the palpebral lobe and penetrate the conjunctiva of the lateral superior fornix, approximately 4 to 5 mm above the superior tarsal margin.
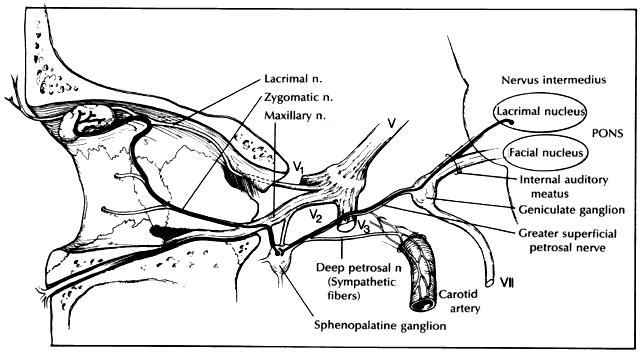
Innervation of the lacrimal gland is quite complex (Fig. 1). Parasympathetic motor fibers originate from the lacrimal nucleus in the pons. From the nucleus, fibers exit the pons in the ventrolateral aspect of the cerebellopontine angle as the nervus intermedius. This fine nerve runs between the motor root of the seventh and eighth cranial nerves as they enter the internal auditory meatus. The fibers continue through the geniculate ganglion without synapsing and then course away from the seventh cranial nerve. Once the fibers leave the geniculate ganglion they are referred to as the greater superficial petrosal nerve. This nerve runs in a groove in front of the petrous temporal bone. Under the gasserian ganglion it joins the deep petrosal nerve (which consists of sympathetic fibers from the internal carotid plexus) and forms the vidian nerve in the cartilage over the foramen lacerum. The vidian nerve passes through the pterygoid canal to synapse in a ganglion within the pterygopalatine fossa known as the sphenopalatine ganglion, Meckel's ganglion, or pterygopalatine ganglion. The ganglion is attached to the maxillary nerve by several short pterygopalatine nerves that carry the postsynaptic parasympathetic lacrimal fibers. These fibers then enter the orbit via the zygomatic nerve. A branch from the zygomatic nerve leaves the infraorbital groove to join the lacrimal nerve, a branch of the first division of the trigeminal nerve. The lacrimal nerve bifurcates as it enters the posterior edge of the lacrimal gland. The branch containing fibers to the lacrimal gland penetrates to the hilus of the gland and then passes peripherally with the ductules to innervate individual lobules of acini. The other branch of the bifurcation passes forward to provide innervation to the upper eyelid and superior conjunctival fornix.
Sympathetic innervation is known to reach the lacrimal gland via the vidian nerve, described above, as well as via the ophthalmic artery and lacrimal artery. The role of sympathetic innervation in the control of lacrimal secretion remains uncertain.
The lacrimal gland receives its blood supply from the internal and external carotid system. The majority of blood is provided by the internal carotid system via the lacrimal artery, a branch of the ophthalmic artery. The lacrimal artery anastomoses with branches of the external carotid system at a minimum of two sites. The first anastomosis is at the distal tip of the superior orbital fissure with the anterior division of the middle meningeal artery. The second anastomosis is within the eyelids between the anterior deep temporal artery and the lacrimal artery. Occasionally, the infraorbital artery sends a branch to the lacrimal gland, thus establishing a third potential anastomosis between the internal and external carotid system to supply blood to the main lacrimal gland. Venous return from the lacrimal gland drains into the superior ophthalmic vein. Lymphatic drainage parallels the conjunctival system to the preauricular nodes.