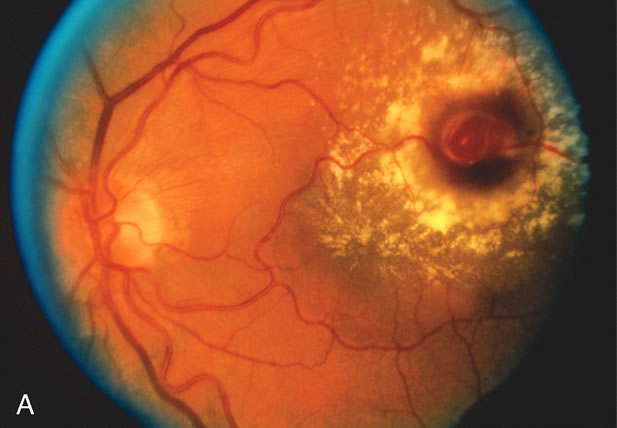
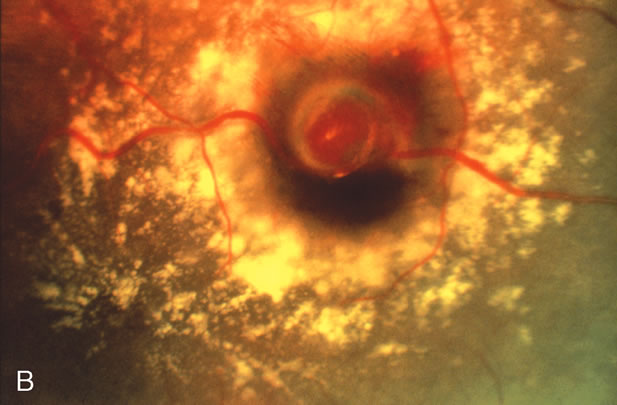
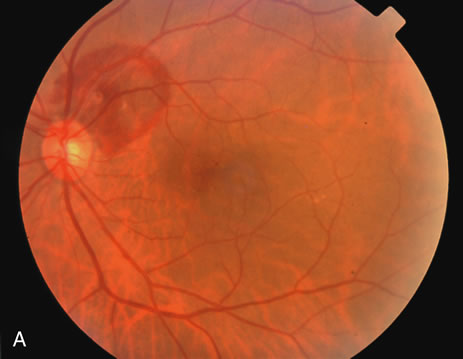
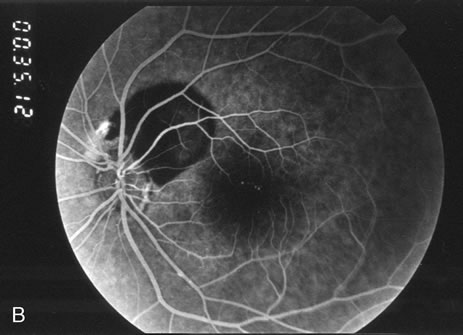
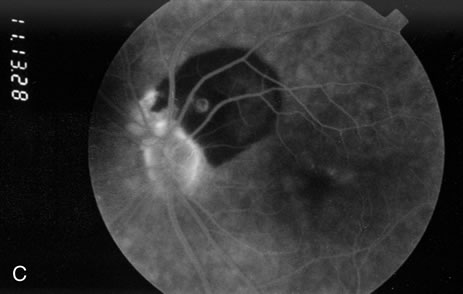
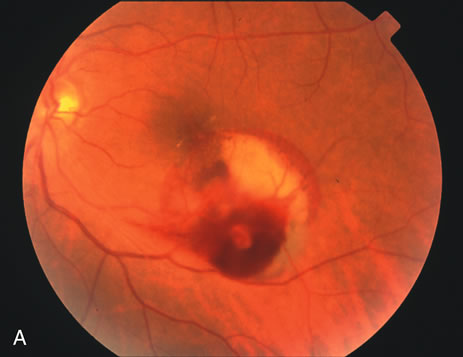
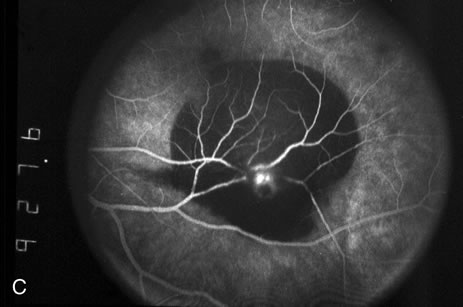
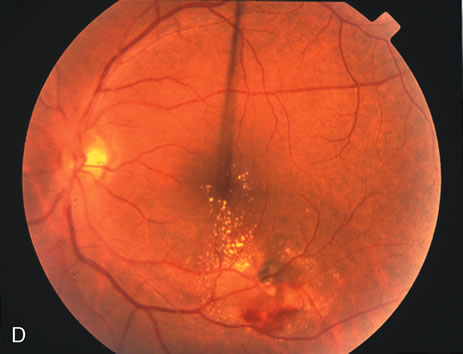
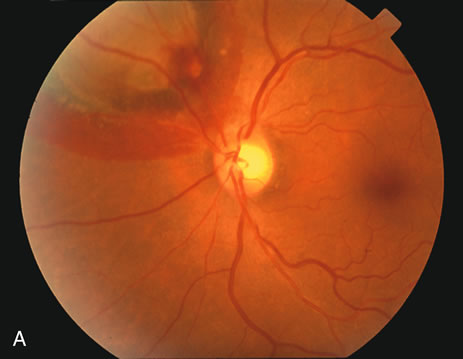
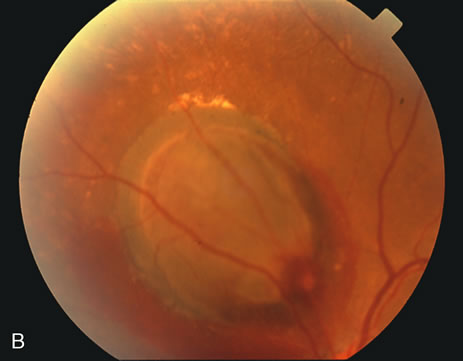
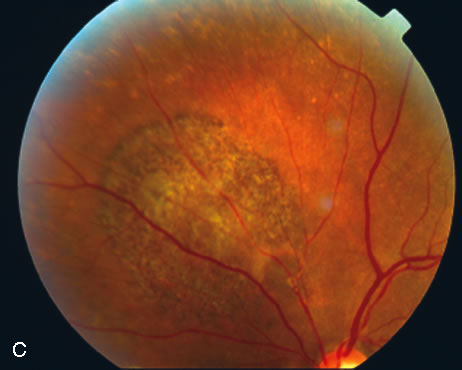
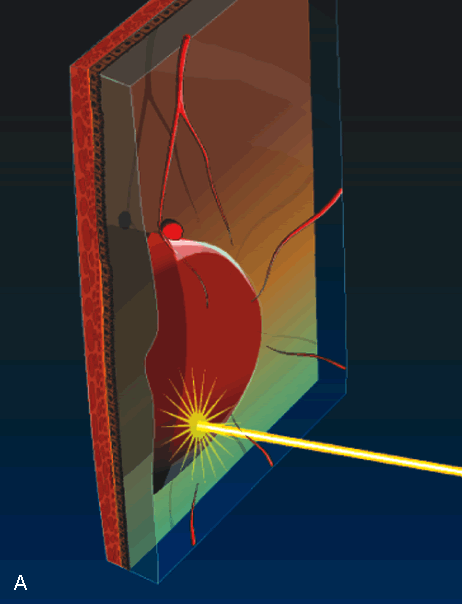
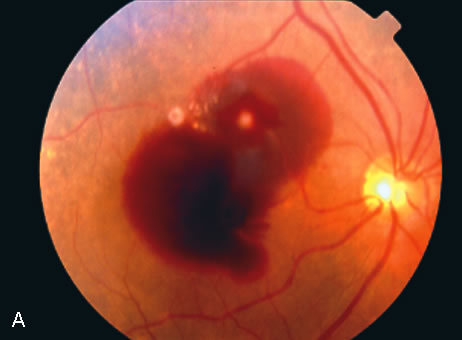
1. Robertson DM: Macroaneurysms of the retinal arteries. Trans Am Acad Ophthalmol Otolaryngol 77:OP55, 1973. 2. Palestine AG, Robertson DM, Goldstein BG: Macroaneurysms of the retinal arteries. Am J Ophthalmol 93:164, 1982. 3. Brown GC, Weinstock F: Arterial macroaneurysm on the optic disc presenting as a mass lesion. Ann Ophthalmol 17:519, 1985. 4. Giuffre G, Montalto FP, Amodei G: Development of an isolated retinal macroaneurysm of the cilioretinal artery. Br J Ophthalmol 71:445, 1987. 5. Lavin MJ, Marsh RJ, Richardson J: Retinal macroaneurysm: Natural history and guidelines for treatment. Br J Ophthalmol 71:817, 1987. 6. Wiznia RA: Development of a retinal artery macroaneurysm at the site of a previously
detected retinal artery embolus. Am J Ophthalmol 114:642, 1982. 7. Ohga H, Egi K, Katayama T, et al: A case of retinal macroaneurysm with congenital ocular toxoplasmosis. Folia Ophthalmol Jpn 41:898, 1990. 8. Panton RW, Goldberg MF, Farber MD: Retinal arterial macroaneurysms: Risk factors and natural history. Br J Ophthalmol 74:595, 1990. 9. Lewis RA, Norton MH, Wise GN: Acquired arterial macroaneurysms of the retina. Br J Ophthalmol 60:21, 1976. 10. Abdel-Khalek MN, Richardson J: Retinal macroaneurysm: Natural history and guidelines for treatment. Br J Ophthalmol 70:2, 1986. 11. Palestine AG, Robertson DM, Goldstein BG: Macroaneurysms of the retinal arteries. Am J Ophthalmol 93:164, 1982. 12. Brown DM, Sobol WM, Folk JC, et al: Retinal arteriolar macroaneurysms: Long term visual outcome. Br J Ophthalmol 78:534, 1994. 13. Tassignon MJ, Stempels N, Van Mulders L: Retrohyaloid premacular hemorrhage treated by q-switched Nd-YAG
laser. Graefes Arch Clin Exp Ophthalmol 227:440, 1989. 14. Raymond LA: Neodynium:YAG laser treatment for hemorrhages under the internal limiting
membrane and posterior hyaloid face in the macula. Ophthalmology 102:406, 1995. 15. Johnson MW, Olsen KR, Hernandez E: Tissue plasminogen activator treatment of experimental subretinal hemorrhage. Retina 11:250, 1991. 16. Sanders D, Peyman GA, Fishman G, et al: The toxicity of whole blood and hemoglobin. Graefes Arch Clin Exp Ophthalmol 197:255, 1975. 17. Toth CA, Morse LS, Hjelmeland LM, el al: Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol 109:723, 1991. 18. Ohji M, Saito Y, Lewis JM, et al: Pneumatic displacement of subretinal hemorrhage without tissue plasminogen
activator. Arch Ophthalmol 116:1326, 1998. 19. Hassan AS, Johnson MW, Schneiderman TF, et al: Management of submacular hemorrhage with intravitreal tissue plasminogen
activator injection and pneumatic displacement. Ophthalmology 106:1900, 1999. 20. Lewis H: Intraoperative fibrinolysis of submacular hemorrhage with tissue plasminogen
activator and surgical drainage. Am J Ophthalmol 118:559, 1994. 21. Hanscom TA, Diddie KR: Early surgical drainage of macular subretinal hemorrhage. Arch Ophthalmol 105:1722, 1987. 22. Peyman GA, Nelson NC, Alturki W, et al: Tissue plasminogen activating factor assisted removal of subretinal hemorrhage. Ophthalmic Surg 22:575, 1991. 23. Moriarty AP, McAllister IL, Constable IJ: Initial clinical experience with tissue plasminogen activator (tPA) assisted
removal of submacular hemorrhage. Eye 9:582, 1995. 24. Lim JI, Drews-Botsch C, Sternberg P, et al: Submacular hemorrhage removal. Ophthalmology 102:1393, 1995. 25. Ibanez HE, Williams DF, Thomas MA, et al: Surgical management of subretinal hemorrhage: A series of 47 consecutive
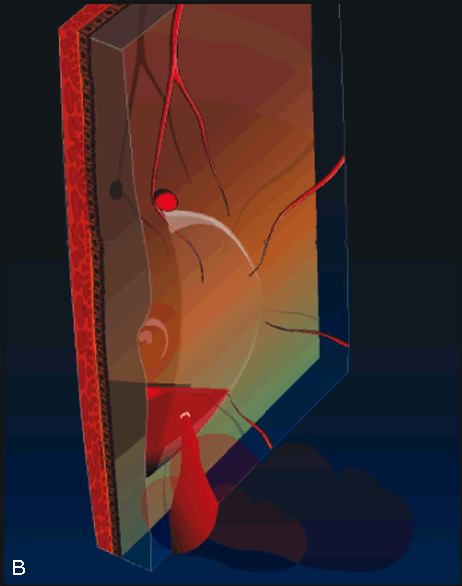
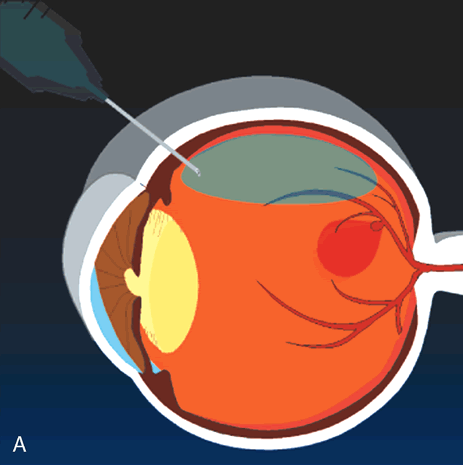
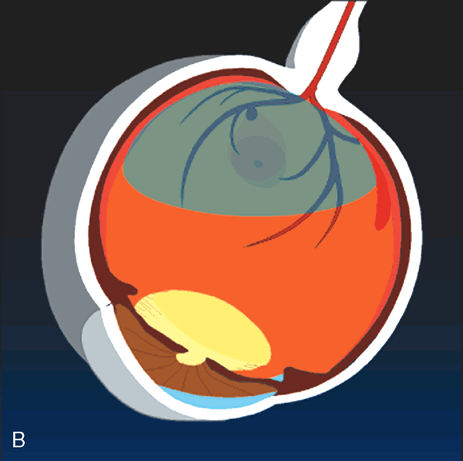
cases. Arch Ophthalmol 113:62, 1995. 26. Brent BD, Gonce M, Diamond JG: Pars plana vitrectomy for complicated of retinal arterial macroaneurysms—a
case series. Ophthalmic Surg 24:534, 1993. |