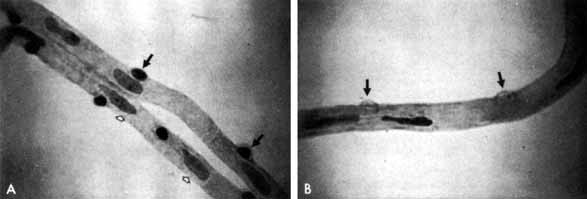
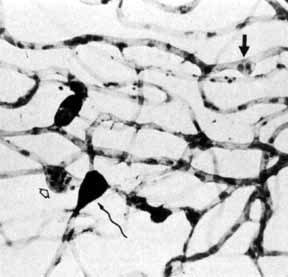
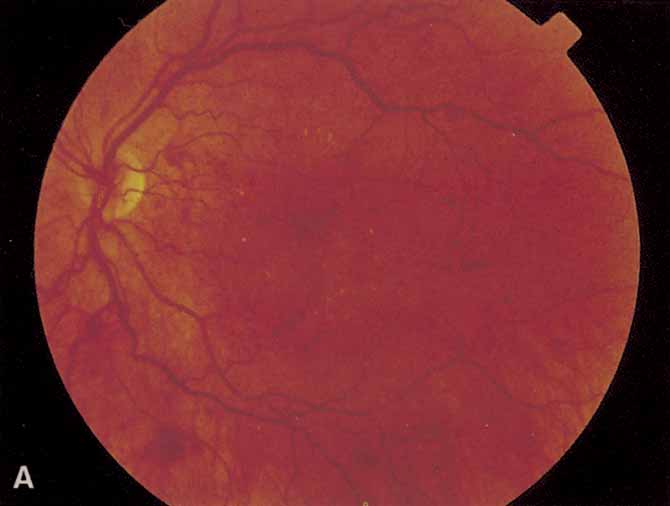
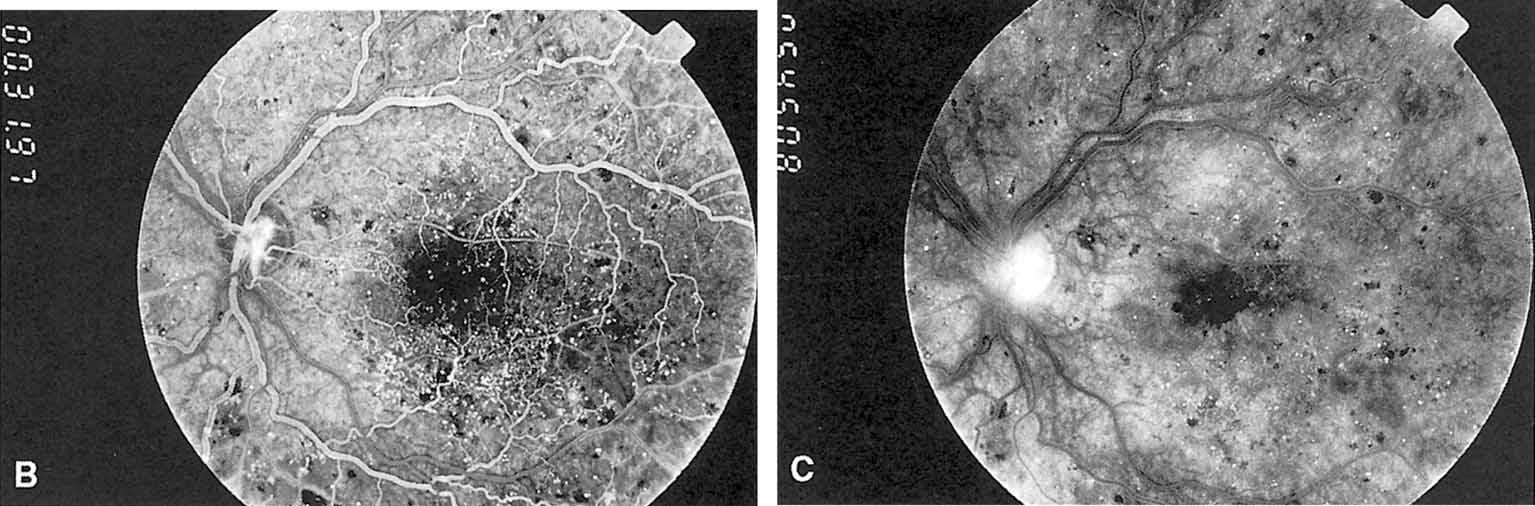
1. Bloodworth JMBJr , Molitor DL: Ultrastructural aspects of human and canine diabetic retinopathy. Invest Ophthalmol 4:1037, 1965 2. Bloodworth JMBJr , Engerman RL: Diabetic microangiography in the experimentally-diabetic dog and
its prevention by careful control with insulin. Diabetes 22:290, 1973 3. Cogan DG, Kuwabara T: The mural cell in perspective. Arch Ophthalmol 78:133, 1967 4. Yanoff M: Ocular pathology of diabetes mellitus. Am J Ophthalmol 67:21, 1969 5. deVenecia G, Davis MD, Engerman R: Clinicopathologic correlations in diabetic retinopathy. Arch Ophthalmol 94:1766, 1976 6. Bresnick GH, Davis MD, Myers FL, et al: Clinicopathological correlations in diabetic retinopathy: II. Clinical
and histologic appearances of retinal capillary microaneurysms. Arch Ophthalmol 95:1215, 1977 7. Schatz H, Patz A: Cystoid maculopathy in diabetics. Arch Ophthalmol 94:761, 1976 8. Fark`s TG, Sylvester V, Archer D: An electron microscopic study of the choriocapillaries and Bruch's
membrane in diabetic retinopathy. Trans Ophthalmol Soc UK 90:657, 1977 9. Hersh PS, Green WR, Thoms JJV: Tractional venous loops in diabetic retinopathy. Am J Ophthalmol 92:661, 1981 10. Early Treatment Diabetic Retinopathy Study Research Group: Fundus photographic
risk factors for progression of diabetic retinopathy. Ophthalmology 98:823, 1991 11. Early Treatment Diabetic Retinopathy Study Research Group: Early Photocoagulation
for diabetic retinopathy. ETDRS Report Number 9. Ophthalmology 98:766, 1991 12. Shimuzu K, Kobayashi Y, Muraoka K: Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology 88:601, 1981 13. Valsania P, Warram JH, Rand LI, et al: Different determinants of neovascularization on the optic disc and on the
retina in patients with severe nonproliferative diabetic retinopathy. Arch Ophthalmol 111:202, 1993 14. Foos RY, Kreiger AE, Forsxthe AB, et al: Posterior vitreous detachment in diabetic subjects. Ophthalmology 87:122, 1980 15. Davis MD: Vitreous contraction in proliferative diabetic retinopathy. Arch Ophthalmol 74:741, 1965 16. Anderson BJr : Activity and diabetic vitreous hemorrhage. Ophthalmology 87:173, 1980 17. Tasman W: Diabetic vitreous hemorrhage and its relationship to hypoglycemia. Mod Prob Ophthalmol 20:413, 1979 18. Ralsay RC, Knobloch WH, Cantrill HL: Timing of vitrectomy for active proliferative diabetic retinopathy. Ophthalmology 93:283, 1986 19. O'Hanley GP, Canny CLB: Diabetic dense premacular hemorrhage. A possible indication for prompt
vitrectomy. Ophthalmology 92:507, 1985 20. Bresnick GH, Haight B, deVenecia G: Retinal wrinkling and macular heterotopia in diabetic retinopathy. Arch Ophthalmol 97:1890, 1979 21. Kostraaba JN, Dorman JS, Orchard TJ, et al: Contribution of diabetes duration before puberty to development of microvascular
complications in IDDM subjects. Diabetes Care 12:686, 1989 22. Klein R, Klein BEK, Syrjala SE, et al: Wisconsin epidemiologic study of diabetic retinopathy. In Friedman EA, L'Esperance FA, eds. Diabetic Renal-Retinal Syndrome. New York: Grune & Stratton, 1981:21 23. Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetes retinopathy. III. Prevalence
and risk of diabetic retinopathy when age of diagnosis is 30 or more
years. Arch Ophthalmol 102:527, 1984 24. Sjolie AK: Ocular complications in insulin treated diabetes mellitus. Ophthalmologica. 1981;63:1–76 25. Palmberg P, Smith M, Waltman S, et al: The natural history of retinopathy in insulin-dependent juvenile-onset
diabetes. Ophthalmology 88:613, 1981 26. Dwyer MS, Melton LJ, Ballard DL, et al: Incidence of diabetic retinopathy and blindness: A population-based
study in Rochester, Minoesota. Diabetes Care 8:316, 1985 27. Klein R, Klein BEK: Epidemiology of proliferative diabetic retinopathy. Diabetes Care 15:1875, 1992 28. Klein R, Davis MD, Moss SE, et al: The Wisconsin epidemiologic study of diabetic retinopathy: a comparison
of retinopathy in younger and older onset diabetic persons. In Vranic M, Hollenberg C, Steiner G, eds. Comparison of Type I and Type II Diabetes. New York: Plenum, 1985:321–325 29. Klein R, Klein BE, Moss SE, et al: The Wisconsin Epidemiologic Study of diabetic retinopathy. XIV. Ten-xear
inchdence and progression of diabetic retinopathy. Arch Ophthalmol 112:1217, 1994 30. Yanko L, Goldbourt U, Michaelson IC, et al: Prevalence and 15-year incidence of retinopathy and associated characteristics
in middle-aged and elderly diabetic men. Br J Ophthalmol 67:759, 1983 31. Diabetes Control and Complications Trial Research Group: The effect of
intensive diabetes treatment on the progression of diabetic retinopathy
in insulin-dependent diabetes mellitus. Arch Ophthalmol 113:36, 1995 32. Diabetes Control and Complications Trial Research Group. The effect of
intensive treatment of diabetes on the development and progression of
long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977, 1993 33. United Kingdom Prospective Diabetes Study Group: Intensive blood-glucose
control with sulphonylureas or insulin compared with conventional
treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837, 1998 34. Gray A, Raikou M, McGuire A, et al: Cost effectiveness of an intensive blood glucose control policy in patients
with type 2 diabetes: economic analysis alongside randomised controlled
trial (UKPDS 41). United Kingdom Prospective Diabetes
Study Group. BMJ 320:1373, 2000 35. Brinchmann-Hansen O, Dahl-Jorgensdn K, Sandvik L, et al: Blood glucose concentrations and progression of diabetic retinopathy: the
seven year results of the Oslo study. BMJ 304:19, 1992 36. Lawson PM, Champion MC, Canny C, et al: Continuous subcutaneous insulin infusion (CSII) does not prevent
progression of proliferative and preproliferative retinopathy. Br J Ophthalmol 66:762, 1982 37. Brinchmann-Hansen O, Dami-Jorgensen K, Hanssen KF, et al: Effects of intensified insulin treatment on various lesions of diabetic
retinopathy. Am J Ophthalmol 100:644, 1985 38. vanBallegooie E, Hooymans JM, Timmerman Z, et al: Rapid deterioration of diabetic retinopathy during treatment with continuous
subcutaneous insulin infusion. Diabetes Care 7:236, 1984 39. KROC Collaborative Study Group: Diabetic retinopathy after two years of
intensified insulin treatment. JAMA 260:37, 1988 40. Ramsay RC, Goetz FC, Sutherland DER, et al: Progression of diabetic retinopathy after pancreas transplantation for
insulin-dependent diabetes mellitus. New Engl J Med 318:208, 1988 41. Chavers BM, Mauer SM, Ramsay RC, et al: Relationship between retinal and glomerular lesions in IDDM patieots. Diabetes 43:441, 1994 42. Orchard TJ, Dorman JS, Maser RE, et al: Factors associated with avoidance of severe complications after 25 yr of
IDDM. Diabetes Care 13:741, 1990 43. Parving H-H: Impact of blood pressure and antihypertensive treatment on incipient and
overt nephropathy, retinopathy, and endothelial permeability in diabetes
mellitus. Diabetes Care 14:260, 1991 44. Jerneld B, Algvere P: Proteinuria and blood glucose levels in a population with diabetic retinopathy. Am J Ophthalmol 104:283, 1987 45. Mogensen CE, Vigstrup J, Ehlers N: Microalbuminuria predicts proliferative diabetic retinopathy. Lancet 1:1512, 1985 46. Klein R, Klein BEK, Moss SE, et al: The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence
and risk of diabetic retinopathy when age is less than 30 years. Arch Ophthalmol 102:520, 1984 47. Nelson RG, Wolfe JA, Horton MB, et al: Proliferative retinopathy in NIDDM. Diabetes 38:435, 1989 48. Cruickshanks KJ, Ritter LL, Klein R, et al: The Association of Microalbuminuria with Diabetic Retinopathy. Ophthalmology. 100:862, 1993 49. Feldman JN, Hirsch SR, Beyer MM: Prevalence of diabetic nephropathy at time of treatment for diabetic retinopathy. In Friedman EA, L'Esperance FA, eds. Diabetic Renal-Retinal Syndrome. New York: Grune & Stratton, 1982:9–2050 50. Klein BE, Klein R, Moss SE, et al: A cohort study of the relationship of diabetic retinopathy to blood pressure. Arch Ophthalmol 113:601, 1995 51. Dornan T, Mann JI, Turner R: Factors protective against retinopathy in insulin-dependent diabetes
free of retinopathy for 30 years. Br Med J 285:1073, 1982 52. Gray RS, Starkey IR, Rainbow S, et al: HLA antigens and other risk factors in the development of retinopathy in
type I diabetes. Br J Ophthalmol 66:280, 1982 53. Janka HU, Warram JH, Rand LI, et al: Risk factors for progression of background retinopathy in long-standing
IDDM. Diabetes. 38:460, 1989 54. United Kingdom Prospective Diabetes Study Group: Efficacy of atenolol and
captopril in reducing risk of lacrovascular and microvascular complications
in type 2 diabetes: UKPDS 39. BMJ 317:713, 1998 55. Chaturvedi N, Sjolie AK, Stephenson JM, et al: Effect of lisinopril on progression of retinopathy in normotensive people
with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial
of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet 351:28, 1998 56. Phelps RL, Sakol P, Metzger BE, et al: Changes in diabetic retinopathy during pregnancy: correlations with regulation
of hyperglycemia. Arch Ophthalmol 104:1806, 1986 57. Beethem WP: Diabetic retinopathy in pregnancy. Trans Am Ophthalmol Soc 48:205, 1950 58. Axer-Siegel R, Hod M, Fink-Cohen S, et al: Diabetic retinopathy during pregnancy. Ophthalmology 103:1815, 1996 59. Rosenn B, Miodovnik M, Kranias G, et al: Progression of diabetic retinopathy in pregnancx: association with hypertension
in pregnancy. Am J Obstet Gynecol 166:1214, 1992 60. Klein BEK, Moss SE, Klein R: Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care 13:34, 1990 61. Ohrt V: The influence of pregnancy on diabetic retinopathy with special regard
to the reversible changes shown in 100 pregnancies. Acta Ophthalmol 62:603, 1984 62. Moloney JBM, Drury MI: The effect of pregnancy on the natural course of diabetic retinopathy. Am J Ophthalmol 83:745, 1982 63. Laatikainen L, Larinakir J, Teralo K, et al: Occurrence and prognostic significance of retinopathy in diabetic pregnancy. Metab Pediatr Ophthalmol 4:191, 1980 64. Horvat M, MacLean H, Goldberg L, et al: Diabetic retinopathy in pregnancy, a 12-year prospective study. Br J Ophthalmol 64:398, 1980 65. Dibble CM, Kochenour NK, Worley RJ, et al: Effect of pregnancy on diabetic retinopathy. Obstet Gynecol 49:699, 1982 66. Hercules BL, Wozencroft M, Gayed I, et al: Peripheral retinal ablation in the treatment of proliferative diabetic
retinopathy during pregnancy. Br J Ophthalmol 64:87, 1980 67. Coustan DR: Pregnancy in diabetic women. N Engl J Med 319:1663, 1988 68. Sinclair SH, Nesler C, Foxman B, et al: Macular edema and pregnancy in insulin-dependent diabetes. Am J Ophthalmol 97:154, 1984 69. Harris EL, Feldman S, Robinson CR, et al: Racial differences in the relationship between blood pressure and risk
of retinopathy among individuals with NIDDM. Diabetes Care 16:748, 1993 70. Raab MF, Gagliano DA, Sweeney HE: Diabetic retinopathy in blacks. Diabetes Care 13:1202, 1990 71. Arfken CL, Salicrup AE, Meuer SM, et al: Retinopathy in African Americans and whites with insulin-dependent
diabetes mellitus. Arch Intern Med 154:2597, 1994 72. Moss SE, Klein R, Klein BEK: Cigarette smoking and ten-year progression of diabetic retinopathy. Ophthalmology 103:1438, 1996 73. Chew EY, Klein ML, Ferris FL, et al: Association of elevated serum lipids with retinal hard exudate in diabetic
retinopathy. Arch Ophthalmol 114:1079, 1996 74. Varma SD: Aldose reductase and the etiology of diabetic cataracts. Curr Top Eye Res 3:91, 1980 75. Kador PF, Kinoshita JF, Sharpless NE: Aldose reductase inhibitors: A potential new class of agents for the pharmacological
control of certain diabetic complications. J Med Chem 28:841, 1985 76. Chylack LT, Henriques HF, Cheng HM, et al: Efficacy of alrestatin, an aldose reductase inhibitor, in human diabetic
and nondiabetic lenses. Ophthalmology 86:1579, 1979 77. Lightman S, Rechthand E, Terubayashi H, et al: Permeability changes in blood-retinal barrier of galactosemic rats
are prevented by aldose reductase inhibitors. Diabetes 36:1271, 1987 78. Robison W, Kador P, Kinoshita J: Retinal capillaries: basement membrane thickening by galactosemia prevented
with aldose reductase inhibitor. Science 220:1177, 1983 79. Sosula L, Beaumont P, Hollows FC, et al: Dilatation and endothelial proliferation of retinal capillaries in streptozotocin
diabetic rats: quantitative electron microscopy. Invest Ophthalmol 11:926, 1972 80. Kador PF, Akagi Y, Terubayashi H, et al: Prevention of pericyte ghost formation in retinal capillaries of galactose-fed
dogs by aldose reductase inhibitors. Arch Ophthalmol 106:1099, 1988 81. Judzewitsch RG, Jaspan JB, Polonsky KS, et al: Aldose reductase inhibition improves nerve condhtion velocity in diabetic
patieots. N Engl J Med 308:119, 1983 82. Notvest RR, Inserra JJ: Tolrestat, an aldose reductase inhibitor, prevents nerve dysfunction in
conscious diabetic rats. Diabetes 36:500, 1987 83. Handelsman DJ, Turtle JR: Clinical trial of an aldose reductase inhibitor in diabetic neuropathy. Diabetes 30:459, 1981 84. Varagiannis E, Beyer-Mears A, Cruz E: Diminished proteinuria by an aldose reductase inhibitor, sorbinil. Diabete 33:43A, 1984 85. Williamson JR, Chang K, Tilton RG, et al: Increased vascular permeability in spontaneously diabetic BB/W rats
and in rats with mild versus severe Streptozotocin-induced diabetes. Prevention
by aldose reductase inhhbitors and castration. Diabetes 36:813, 1987 86. Griffith SP, Freeman WL, Shaw CJ, et al: Screening for diabetic retinopathy in a clinical setting: a comparison
of direct ophthalmoscopy by primary care physicians with fundus photography. J Fam Pract 37:49, 1993 87. Sorbinil Retinopathy Trial Research Group: A randomized trial of sorbinil, an
aldose reductase inhibitor, in diabetic retinopathy. Arch Ophthalmol 108:1234, 1990 88. Sorbinil Retinopathy Trial Research Group: The sorbinil retinopathy trial: neuropathy
results. Neurology 43:1141, 1993 89. Frank RN: The aldose reductase controversy. Diabetes 43:169, 1994 90. Michaelson IC: Retinal Circulation in Man and Animals. Springfield, IL: Thomas, 1954:118–131 91. Ashton N: Retinal vascularization in health and disease. Am J Ophthalmol 44:7, 1957 92. Ashton N: Studies of the retinal capillaries in relation to diabetic and other retinopathies. Br J Ophthalmol 47:521, 1963 93. Kohner DM, Shilling JS, Hamilton AM: The role of avascular retina in new vessel formation. Metab Ophthalmol 1:15, 1976 94. Forrester JV, Shafiee A, Schroder S, et al: The role of growth factors in proliferative diabetic retinopathy. Eye 7:276, 1993 95. Aiello LP, Avery RP, Arrigg PG, et al: Vascular endothelial growth factor in ocular fluid of patients with diabetic
retinopathy and other retinal disorders. N Engl J Med 331:1480, 1994 96. Adamis AP, Miller JW, Bernal MT, et al: Increased vascular endothelial growth factor levels in the vitreous of
eyes with proliferative diabetic retinopathy. Am J Ophthalmol 118:445, 1994 97. Malecaze F, Clamens S, Simorre-Pinatel V, et al: Detection of vascular endothelial growth factor messenger RNA and vascular
endothelial growth factor-like activity in proliferative diabetic
retinopathy. Arch Ophthalmol 112:1476, 1994 98. Tolentino MJ, Miller JW, Gragoudas ES, et al: Intravitreous injections of vascular endothelial growth factor produce
retinal ischemia and microangiopathy in an adult primate. Ophthalmology 103:1820, 1996 99. Poulsen JE: Recovery from retinopathy in a case of diabetes with Simmonds' disease. Diabetes 2:7, 1953 100. Alzaid AA, Dinneen SF, Melton LJ III , et al: The role of growth hormone in the development of diabetic retinopathy. Diabetes Care 17:531, 1994 101. Dobbie JG, Kwaan HC, Colwell J, et al: Role of platelets in pathogenesis of diabetic retinopathy. Arch Ophthalmol 91:107, 1974 102. Bensoussan D, Levy-Toledano S, Passa P, et al: Platelet hyperaggregation and increased plasma level of von Willebrand
factor in diabetics with retinopathy. Diabetologia 11:307, 1975 103. Colwell J: Inhibition of LASS and platelet aggregation in early diabetes mellitus. Diabetes 27:684, 1975 104. Segal J, Colwell J, Crook L, et al: Increased platelet aggregation in early diabetes mellitus. Ann Intern Med 82:733, 1975 105. Ho PC, Feman SS, Stein RS, et al: Proliferative diabetic retinopathy in patients with defects of platelet
function. Am J Ophthalmol 88:37, 1979 106. Dintenfass L: Blood viscosity factors in severe nondiabetic and diabetic retinopathy. Biorheology 14:151, 1977 107. Little HL, Sachs AH: Role of abnormal blood rheology in the pathogenesis of diabetic retinopathy. Trans Am Acad Ophthalmol Otolaryngol 83:522, 1977 108. McMillan DE, Utterback NG, LaPoma J: Reduced erythrocyte deformability in diabetes. Diabetes 27:895, 1978 109. Chew EY, Klein ML, Murphy RP, et al: Effects of aspirin on vitreous/preretinal hemorrhage in patients with
diabetes mellitus. ETDRS Report No. 20. Arch Ophthalmol 113:52, 1995 110. DAMAD Study Group: Effect of aspirin alone and aspirin plus Dipyridamole
in early diabetic retinopathy. A multicenter randomized controlled clinical
trial. Diabetes 38:481, 1989 111. Ticlopidine Microangiopathy of Diabetes Study Group: Ticlopidine treatment
reduces the progression of nonproliferative diabetic retinopathy. Arch Ophthalmol 108:1577, 1990 112. Sonkin PL, Kelly LW, Sinclair SH, et al: Pentoxifylline increases retinal capillary blood flow velocity in patients
with diabetes. Arch Ophthalmol 111:1647, 1993 113. Cambie E: Functional results following argon laser photocoagulation in eyes with
diabetic retinopathy. In Friedman EA, L'Esperance FA, eds. Diabetic Renal-Retinal Syndrome. New York: Grune & Stratton, 1980:295–307 114. Gliem H, Schulze DP: Sofortadaptation, Blendungsupfindlichkeitr und diabetische retinopathie. Klin Monatsbl Augenheilkd 166:766, 1978 115. Henson DB, North RV: Dark adaptation in diabetes mellitus. Br J Ophthalmol 63:539, 1979 116. Pender PM, Benson WE, Compton H, et al: The effects of panretinal photocoagulation on dark adaptation in diabetics
with proliferative retinopathy. Ophthalmology 88:634, 1981 117. Kinnear PR, Aspinall PA, Lakowski R: The diabetic eye and colour vision. Trans Ophthalmol Soc UK 92:69, 1972 118. Graham K, Keson CM, Kennedy HB, et al: Relevance of colour diabetic retinopathy to self-monitoring of blood
glucose. Br Med J 281:971, 1980 119. Moloney J, Drury MI: Retinopathy and retinal function in insulin-dependent diabetes mellitus. Br J Ophthalmol 66:759, 1982 120. Roy MS, McCulloch C, Hanna AK, et al: Colour vision in long-standing diabetes mellitus. Br J Ophthalmol 68:215, 1984 121. Sokol S, Moskowitz A, Sharf B, et al: Contrast sensitivity in diabetics with and without background retinopathy. Arch Ophthalmol 103:51, 1985 122. Trick GL, Burde RM, Gordon ME, et al: The relationship between hue discrimination and contrast sensitivity deficits
in patients with diabetes mellitus. Ophthalmology 95:693, 1988 123. Ghafour IM, Foulds WS, Allan D, et al: Contrast sensitivity in diabetic subjects with and without retinopathy. Br J Ophthalmol 66:492, 1982 124. Vesti E, Trick GL: Diabetes can alter the interpretation of visual dysfunction in ocular hypertension. Ophthalmology 103:1419, 1996 125. Simonsen SE: ERG in diabetes. In Francois J, ed. The Clinical Value of Electroretinography. New York: S Karger, 1968:403 126. Simonsen SE: The value of the oscillatory potential in selecting juvenile diabetics
at risk of developing proliferative retinopathy. Dev Ophthalmol 2:222, 1981 127. Frost-Larson K, Larson HW, Simonsen SE: Value of electroretinography and dark adaptation as prognostic tools in
diabetic retinopathy. Dev Ophthalmol 2:222, 1981 128. Bresnick GL, Korth K, Groo A, et al: Electroretinographic oscillatory potentials predict progression of diabetic
retinopathy. Preliminary report. Arch Ophthalmol 102:1307, 1984 129. Algvere P, Gjotterberg M: Diagnostic value of oscillatory potentials of the electroretinogram and
fluorescein angiography in diabetic proliferative retinopathy. Ophthalmologica 168:97, 1974 130. Bresnick GH, Palta M: Oscillatory potenthal amplitudes. Ophthalmology 105:929, 1987 131. Benson WE, Brown GC, Tasman W: Diabetes and Its Ocular Complications. Philadelphia: WB Saunders, 1988:90–109 132. Aiello LM, Beetham WP, Balodimos MD, et al: Ruby laser photocoagulation in the treatment of diabetic retinopathy, Preliminary
report. In Goldberg MD, Fine SL, eds. Symposium on the Treatment of Diabetic Retinopathy. Washington, DC: US Public Health Service, Publication No. 1890; 1968:187–463 133. Beetham WP, Aiello IL, Balodimos MC, et al: Ruby laser photocoagulation of early diabetic neovascular retinopathy. Arch Ophthalmol 83:261, 1970 134. Duane TD, Behrendt T, Field RA: Net vascular pressure ratios in diabetic retinopathy. In: Goldberg MF, Fine SL, eds. Symposium on the Treatment of Diabetic Retinopathy. Washington, DC: US Public Health Service, Publication 1890; 1968:657 135. Wetzig PC, Jepson CN: Treatment of diabetic retinopathy by light coagulation. Am J Ophthalmol 62:459, 1966 136. Behrendt T, Duane TD: Unilateral complications in diacetic retinopathy. Trans Am Acad Ophthalmol Otolaryngol 74:28, 1970 137. Coop HV: Photocoagulation for proliferative diabetic retinopathy. Trans Ophthalmol Soc N Z ???:31, 1970 138. Duane TD: Is diabetic retinopathy uncontrollable? Am J Ophthalmol 71:286, 1971 139. Ederer F, Hiller R: Clinical trials, diabetic retinopathy and photocoagulation: A reanalysis
of five studies. Surv Ophthalmol 19:267, 1975 140. Diabetic Retinopathy Study Group: Preliminary report on effects of photocoagulation
therapy. Am J Ophthalmol 81:383, 1976 141. Diabetic Retinopathy Study Group: Photocoagulation treatment of proliferative
diabetic retinopathy: the second report of Diabetic Retinopathy
Study findings. Ophthalmology 85:82, 1978 142. Diabetic Retinopathy Study Research Group: Four risk factors for severe
visual loss in diabetic retinopathy: the third report from the Diabetic
Retinopathy Study. Arch Ophthalmol 97:654, 1979 143. Patel V, Rassam S, Newsom R, et al: Retinal blood flow in diabetic retinopathy. BMJ 305:678, 1992 144. Grunwald JE, Brucker AJ, Petrig BL, et al: Retinal blood flow regulation and the clinical response to panretinal photocoagulation
in proliferative diabetic retinopathy. Ophthalmology 96:1518, 1989 145. Glaser BM, Campochiaro PA, Davis DL Jr , et al: Retinal pigment epithelial cells release an inhibitor of neovascularization. Arch Ophthalmol 103:1870, 1985 146. Stefansson E, Landers MBIII , Wolbarsht ME: Oxygenation and vasodilation in relation to diabetic and other proliferative
retinopathies. Ophthalmic Surg 17:208, 1983 147. Wolbarsht ML, Landers MBIII , Stefansson E: Vasodilation and the etiology of diabetic retinopathy: a new model. Ophthalmic Surg 12:104, 1981 148. Wolbarsht ML, Landers MB: The rationale of photocoagulation therapy for proliferative diabetic retinopathy: a
review and a model. Ophthalmic Surg 11:235, 1980 149. Stefansson E, Machemer R, de Juan E Jr , et al: Retinal oxygenation and laser treatment in patients with diabetic retinopathy. Am J Ophthalmol 113:36, 1992 150. Schiodte N: Ocular effects of panretinal photocoagulation. Acta Ophthalmol 66(suppl):9, 1988 151. Marshall J, Clover G, Rothery S: Some new findings on retinal irradiation by krypton and argon lasers. Doc Ophthalmol 36:21, 1984 152. Singh A, Boulton M, Lane C, et al: Inhibition of microvascular endothelial cell proliferation by vitreous
following retinal scatter photocoagulation. Br J Ophthalmol 74:328, 1990 153. Lobes LAJr , Benson WE, Grand MG: Panfunduscope contact lens for argon laser therapy. Ann Ophthalmol 13:713, 1981 154. Frank RN: Visual fields and electroretinography following extensive photocoagulation. Arch Ophthalmol 93:591, 1975 155. Ogden TE, Riekolf FT, Benkwith SM: Correlation of histologic and electroretinographic changes in peripheral
retinal ablation in the rhesus monkey. Am J Ophthalmol 81:272, 1976 156. Krypton Argon Regression Neovascularization Study Research Group: Randomized
comparison of krypton versus argon scatter photocoagulation for
diabetic disc neovascularization. Ophthalmology 100:1655, 1993 157. Aylward GW, Pearson RV, Jagger JD, et al: Extensive argon laser photocoagulation in the treatment of proliferative
diabetic retinopathy. Br J Ophthalmol 73:197, 1989 158. Singerman LJ: PDR in juvenile onset diabetics: high-risk proliferative diabetic
retinopathy in juvenile onset diabetics. Retina 1:18, 1981 159. Vine AK: The efficacy of additional argon photocoagulation for persistent, severe
proliferative diabetic retinopathy. Ophthalmology 92:1532, 1985 160. Reddy VM, Zamora RL, Olk RJ: Quantitation of retinal ablation in proliferative diabetic retinopathy. Am J Ophthalmol 119:760, 1995 161. Doft BH, Metz DJ, Kelsey SF: Augmentation laser for proliferative diabetic retinopathy that fails to
respond to initial panretinal photocoagulation. Ophthalmology 99:1728, 1992 162. Leyers SM: Macular edema after scatter laser photocoagulation for proliferative diabetic
retinopathy. Am J Ophthalmol 90:210, 1980 163. Ferris FL, Podgor MJ, Davis MD: The Diabetic Retinopathy Research Group: macular edema in diabetic retinopathy
study patients. Ophthalmology 94:754, 1987 164. Kleiner RC, Elman MJ, Murphy RP, et al: Transient severe visual loss after panretinal photocoagulation. Am J Ophthalmol 106:298, 1988 165. Miettinen H, Haffner S, Lehto S, et al: Retinopathy predicts coronary heart disease events in NIDDM patients. Diabetes Care 19:1445, 1966 166. Ramsay RC, Cantrill HL, Knobloch WH: Cryoretinopexy for proliferative diabetic retinopathy. Can J Ophthalmol 17:17, 1982 167. Haut J, Robert P, Chatellier PH, et al: Place de la cryotherapie dans le traitement de la retinopathie diabetique. Bull Mem Soc Fr Ophthalmol 90:124, 1978 168. Lempert P: Cryoablation for diabetic retinopathy. Ann Ophthalmol 11:740, 1979 169. Schimek RA, Spencer R: Cryopexy treatment of proliferative diabetic retinopathy. Arch Ophthalmol 97:1276, 1979 170. Oosterhuis JA, Bijlmer-Gortner H: Cryotreatment in proliferative diabetic retinopathy. Arch Ophthalmol 97:1276, 1979 171. Benedett R, Olk JR, Arribas NP, et al: Transconjunctival anterior retinal cryotherapy for proliferative diabetic
retinopathy. Ophthalmology 94:612, 1987 172. Ross WH, Gottner MJ: Peripheral retinal cryopexy for subtotal vitreous hemorrhage. Am J Ophthalmol 105:377, 1988 173. Daily MJ, Gieser RG: Treatment of proliferative diabetic retinopathy with panretinal cryotherapy. Ophthalmic Surg 9:741, 1984 174. Patz A, Schatz H, Berkow JW, et al: Macular edema: an overlooked complication of diabetic retinopathy. Trans Am Acad Ophthalmol Otolaryngol 77:34, 1973 175. Early Treatment Diabetic Retinopathy Study Research Group: Focal photocoagulation
treatment of diabetic macular edema. Relationship of treatment
effect to fluorescein angiographic and other retinal characteristics
at baseline: ETDRS report no. 19. Arch Ophthalmol 113:1144, 1995 176. Striph GG, Hart WM, Olk RJ: Modified grid laser photocoagulation for diabetic macular edema. Ophthalmology 95:1673, 1988 177. Ticho U, Patz A: The role of capillary perfusion in the management of diabetic macular edema. Am J Ophthalmol 76:880, 1973 178. Martidis A, Duker JS, Greenberg PB, et al: Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 109:920, 2002 179. Jonas JB, Kreissig I, Sofker A, et al: Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol 121:57, 2003 180. Benz MS, Murray TG, Dubovy SR, et al: Endophthalmitis caused by Mycobacterium chelonae abscessus after intravitreal
injection of triamcinolone. Arch Ophthalmol 121:271, 2003 181. Nelson M, Tennant M, Sivalingam A, et al: Infectious and presumed noninfectious endophthalmitis after intravitreal
triamcinolone acetonide injection. Retina 23:686, 2003 182. Harbour JW, Smiddy WE, Flynn HW Jr , et al: Vitrectomy for diabetic macular edema associated with a thickened and taut
posterior hyaloid membrane. Am J Ophthalmol 121:405, 1996 183. Lewis H, Abrams GW, Blumenkranz MS, et al: Vitrectomy for diabetic macular traction and edema associated with posterior
hyaloidal traction. Ophthalmology 99:753, 1992 184. Pendergast SD, Hassan TS, Williams GA, et al: Vitrectomy for diffuse diabetic macular edema associated with a taut premacular
posterior hyaloid. Am J Ophthalmol 130:178, 2000 185. Gandorfer A, Messmer EM, Ulbig MW, et al: Resolution of diabetic macular edema after surgical removal of the posterior
hyaloid and the inner limiting membrane. Retina 20:126, 2000 186. Nasrallah FP, Jalkh AE, Van Coppenolle F, et al: The role of the vitreous in diabetic macular edema. Ophthalmology 95:1335, 1988 187. Tachi N, Ogino N: Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol 122:258, 1996 188. Otani T, Kishi S: A controlled study of vitrectomy for diabetic macular edema. Am J Ophthalmol 134:21, 2002 189. Ferris FL III , et al: How effective are treatments for diabetic retinopathy? JAMA 269:1290, 1993 190. Javitt JC, Aiello LP, Bassi LJ, et al: Detecting and treating retinopathy in patients with type I diabetes mellitus. Ophthalmology 98:1565, 1991 191. Rand LI: Financial Implications of implementing standards of care for diabetic eye
disease. Diabetes Care 15:32, 1992 192. Singer DE, Nathan DM, Fogel HA, et al: Screening for diabetic retinopathy. Ann Intern Med 116:660, 1992 193. Klein R: Eye-care delivery for people with diabetes. Diabetes Care 17:614, 1994 194. Wang F, Javitt JC: Eye care for elderly Americans with diabetes mellitus. Ophthalmology 103:1744, 1996 195. American Diabetes Association: Screening for diabetic retinopathy. Diabetes Care 19(Suppl 1):20, 1996 196. Awh CC, Cupples HP, Javitt JC: Improved detection and referral of patients with diabetic retinopathy by
primary care physicians. Effectiveness of education. Arch Intern Med 151:1405, 1991 197. Dasbach EJ, Fryback DG, Newcomb PA, et al: Cost-effectiveness of strategies for detecting diabetic retinopathy. Diabetes Spectrum 5:99, 1992 198. Kalm H: Non-stereo photographic screening in long-term follow-up
for detection of proliferative diabetic retinopathy. Acta Ophthalmol (Copenh) 70:228, 1992 199. Nathan DM, Fogel HA, Godine JE, et al: Role of diabetologist in evaluating diabetic retinopathy. Diabetes Care 14:26, 1991 200. Leese GP, Ahmed S, Newton RW, et al: Use of mobile screening unit for diabetic retinopathy in rural and urban
areas. BMJ 306:187, 1993 201. Kiri A, Dyer DS, Bressler NM, et al: Detection of diabetic macular edema: Nidek 3Dx stereophotography compared
with fundus biomicroscopy. Am J Ophthalmol 122:654, 1996 202. Benson WE: Vitrectomy. In Duane TD, ed. Clinical Ophthalmology. Hagerstown, MD: Harper & Row, 1996:1–33 203. Lewis H, Abrams GW, Blumenkranz MS, et al: Vitrectomy for diabetic macular traction and edema associated with posterior
hyaloidal traction. Ophthalmology 99:753, 1992 204. Harbour JW, Smiddy WE, Flynn HW, et al: Vitrectomy for diabetic macular edema associated with a thickened and taut
posterior hyaloid membrane. Am J Ophthalmol 121:405, 1996 205. Diabetic Retinopathy Vitrectomy Study Research Group: Early vitrectomy
for severe proliferative diabetic retinopathy in eyes with useful vision. Results
of a randomized trial—diabetic retinopathy vitrectomy
study report 3. Ophthalmology 95:1307, 1988 206. Diabetic Retinopathy Vitrectomy Study Research Group: Two year course of
visual acuity in severe proliferative diabetic retinopathy with conventional
management. Ophthalmology 92:492, 1985 207. Diabetic Retinopathy Vitrectomy Study Research Group: Early vitrectomy
for severe vitreous hemorrhage in diabetic retinopathy. Two-year
results of a randomized trial. Diabetic retinopathy vitrectomy study
report 2. Arch Ophthalmol 103:1644, 1985 208. Thompson JT, deBustros S, Michels RG, et al: Results and prognostic factors in vitrectomy for diabetic vitreous hemorrhage. Arch Ophthalmol 105:191, 1987 209. Martin DF, McCuen BW II : Efficacy of fluid-air exchange for postvitrectomy diabetic vitreous
hemorrhage. Am J Ophthalmol 114:457, 1992 210. Charles S, Flinn CE: The natural history of diabetic extramacular traction retinal detachment. Arch Ophthalmol 99:66, 1981 211. Han DP, Murphy ML, Mieler WF: A modified en bloc excision technique during vitrectomy for diabetic traction
retinal detachment. Ophthalmology 101:803, 1994 212. Williams DF, Williams GA, Hartz A: Results of vitrectomy for diabetic traction retinal detachments using the
En Bloc excision technique. Ophthalmology 96:752, 1989 213. Aaberg TM: Pars plana vitrectomy for diabetic traction retinal detachment. Ophthalmology 88:639, 1981 214. Hutton WL, Bernstein I, Fuller D: Diabetic traction retinal detachment. Factors influencing final visual
acuity. Ophthalmology 87:1071, 1980 215. Meredith TA, Kaplan HJ, Aaberg TM: Pars plana vitrectomy techniques for relief of epiretinal traction by membrane
segmentation. Am J Ophthalmol 89:408, 1980 216. Tolentino FI, Freeman HM, Tolentino FL: Closed vitrectomy in the management of diabetic traction retinal detachment. Ophthalmology 87:1078, 1980 217. Rice TA, Michels RG, Rice EF: Vitrectomy for diabetic rhegmatogenous traction retinal detachment. Am J Ophthalmol 95:33, 1983 218. Barrie T, Feretis E, Leaver P, et al: Closed microsurgery for diabetic traction macular detachment. Br J Ophthalmol 66:754, 1982 219. Han DP, Pulido JS, Mieler WF, et al: Vitrectomy for proliferative diabetic retinopathy with severe equatorial
fibrovascular proliferation. Am J Ophthalmol 119:563, 1995 220. Brown GC, Tasman WS, Benson WE, et al: Reoperation following diabetic vitrectomy. Arch Ophthalmol 110:506, 1992 221. Blankenship GW: Posterior retinal holes secondary to diabetic retinopathy. Arch Ophthalmol 101:885, 1983 222. Rice TA, Michels RG, Rice EF: Vitrectomy for diabetic traction retinal detachment involving the macula. Am J Ophthalmol 95:12, 1983 223. Yeo JH, Glaser BM, Michels RG: Silicone oil in the treatment of complicated retinal detachments. Ophthalmology 94:1109, 1987 224. McCuen BW, Rinkoff JS: Silicone oil for progressive anterior ocular neovascularization after failed
diabetic vitrectomy. Arch Ophthalmol 107:677, 1989 225. Brourman ND, Blumenkranz MS, Cox MS, et al: Silicone oil for the treatment of severe proliferative diabetic retinopathy. Ophthalmology 96:759, 1989 226. Schachat AP, Oyakawa RT, Michels RG, et al: Complications of vitreous surgery from diabetic retinopathy. II. Postoperative
complications. Ophthalmology 90:522, 1983 227. Lewis H, Aaberg TM: Causes of failures after repeat vitreoretinal surgery for recurrent proliferative
vitreoretinopathy. Am J Ophthalmol 111:15, 1991 228. Awasthi P, Sarbhai KP, Maheswari BB, et al: Corneal sensations in diabetes mellitus. Trans III Int Congr (Paris) 1:402, 1974 229. Rogell GD: Incremental panretinal photocoagulation: results in treating proliferative
diabetic retinopathy. Retina 3:308, 1983 230. Schwartz ED: Corneal sensitivity in diabetics. Arch Ophthalmol 91:174, 1974 231. Scullica L, Proto F: Rilievi clinici e statistici sulla sensibilita corneale nei diabetici. Boll Ocul 44:944, 1965 232. Friend J, Snip RC, Kiorpes TC, et al: Metabolic effects of diabetes mellitus on rabbit corneal epithelium. Invest Ophthalmol Vis Sci 19:913, 1980 233. Datiles MB, Kador PF, Fukui HN, et al: Corneal reepithelialization in galactosemic rats. Invest Ophthalmol Clin 24:111, 1984 234. Ohashi Y, Matsuda M, Hosotai H, et al: Aldose reductase inhibitor (CT–112) eyedrops for diabetic
corneal epitheliopathy. Am J Ophthalmol 105:223, 1988 235. Pardos GJ, Kratchmer JH: Comparison of endothelial cell density in diabetics and a control population. Am J Ophthalmol 90:172, 1980 236. Becker B: Diabetes mellitus and primary open angle glaucoma. Trans Am Acad Ophthalmol Otolaryngol 75:293, 1971 237. Tasman W, Magargal LE, Augsburger JJ: Effects of argon laser photocoagulation on rubeosis iridis and angle neovascularization. Ophthalmology 87:400, 1980 238. Jacobson DR, Murphy RP, Rosenthal AR: The treatment of angle neovascularization with panretinal photocoagulation. Ophthalmology 86:1270, 1979 239. Little HL, Rosenthal R, Dellaporta A, et al: The effect of panretinal photocoagulation on rubeosis iridis. Am J Ophthalmol 81:804, 1976 240. Wand M, Dueker DK, et al: Effects of panretinal photocoagulation on rubeosis iridis and neovascular
glaucoma. Am J Ophthalmol 86:332, 1978 241. Mermoud A, Salmon JA, Alexander P, et al: Molteno tube implantation for neovascular glaucoma. Ophthalmology 100:897, 1993 242. Bron AJ, Sparrow J, Brown NAP, et al: The lens in diabetes. Eye 7:260, 1993 243. Ederer F, Hiller R, Taylor HR: Senile lens changes and diabetes in two population studies. Am J Ophthalmol 91:381, 1981 244. Klein BEK, Klein R, Moss RE: Prevalence of cataracts in a population-based study of persons with
diabetes mellitus. Ophthalmology 92:1191, 1985 245. Nielsen NV, Vinding T: The prevalence of cataract in insulin-independent and non-insulin-dependent
diacetes mellitus. Acta Ophthalmol 62:595, 1984 246. Skalka HW, Prchal JT: The effect of diabetes mellitus and diabetic therapy on cataract formation. Ophthalmology 88:117, 1981 247. Klein BE, Klein R, Moss SE: Incidence of cataract surgery in the Wisconsin Epidemiologic Study of Diabetic
Retinopathy. Am J Ophthalmol 119:295, 1995 248. Bernth-Peterson P, Bach E: Epidemiologic aspects of cataract surgery. III: Frequencies of diabetes
and glaucoma in a cataract population. Acta Ophthalmol 61:406, 1983 249. Cunliffe IA, Flanagan DW, George NDL, et al: Extracapsular cataract surgery with lens implantation in diabetics with
and without proliferative retinopathy. Br J Ophthalmol 75:9, 1991 250. Krupsky S, Zalish M, Oliver M, et al: Anterior segment complications in diabetic patients following extracapsular
cataract extraction and posterior chamber intraocular lens implantation. Ophthalmic Surg 22:526, 1991 251. Menchini U, Bandello F, Brancato R, et al: Cystoid macular oedema after extracapsular cataract extraction and intraocular
lens implantation in diabetic patients with diabetes. Br J Ophthalmol 77:208, 1993 252. Squirrell D, Bhola R, Bush J, et al: A prospective, case controlled study of the natural history of diabetic
retinopathy and maculopathy after uncomplicated phacoemulsification cataract
surgery in patients with type 2 diabetes. Br J Ophthalmol 86:565, 2002 253. Zaczek A, Olivestedt G, Zetterstrom C: Visual outcome after phacoemulsification and IOL implantation in diabetic
patients. Br J Ophthalmol 83:1036, 1999 254. Krepler K, Biowski R, Schrey S, et al: Cataract surgery in patients with diabetic retinopathy: visual outcome, progression
of diabetic retinopathy, and incidence of diabetic macular
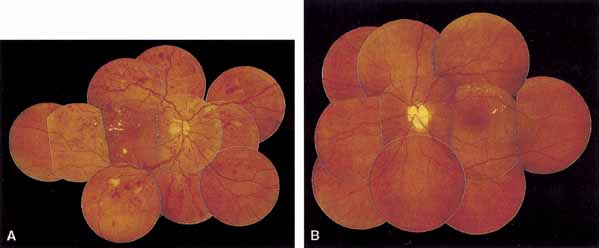
oedema. Graefes Arch Clin Exp Ophthalmol 240:735, 2002 255. Benson WE, Brown GC, Tasman W, et al: Extracapsular cataract extraction with placement of a posterior chamber
lens in patients with diabetic retinopathy. Ophthalmology 100:730, 1993 256. Dowler JG, Sehmi KS, Hykin PG, et al: The natural history of macular edema after cataract surgery in diabetes. Ophthalmology 106:663, 1999 257. Hayashi K, Hayashi H, Nakao F, et al: Posterior capsule opacification after cataract surgery in patients with
diabetes mellitus. Am J Ophthalmol 134:10, 2002 258. Hykin PG, Gregson RMC, Stevens JD, et al: Extracapsular cataract extraction in proliferative diabetic retinopathy. Ophthalmology 100:394, 1993 259. Ruiz RS, Saatci OA: Posterior chamber intraocular lens implantation in eyes with inactive and
active proliferative diabetic retinopathy. Am J Ophthalmol 111:158, 1991 260. Henricsson M, Heijl A, Janzon L: Diabetic retinopathy before and after cataract surgery. Br J Ophthalmol 80:789, 1996 261. Pavese T, Insler MS: Effects of extracapsular cataract extraction with posterior chamber lens
implantation on the development of neovascular glaucoma in diabetics. J Cataract Refract Surg 13:197, 1987 262. Prasad P, Setna PH, Dunne JA: Accelerated ocular neovascularisation in diabetics following posterior
chamber lens implantation. Br J Ophthalmol 74:313, 1990 263. Benson WE, Brown GC, Tasman W, et al: Extracapsular cataract extraction with placement of a posterior chamber
lens in patients with diabetic retinopathy. Ophthalmology 100:730, 1993 264. Jaffe GJ, Burton TC, Kuhn E, et al: Progression of nonproliferative diabetic retinopathy and visual outcome
after extracapsular cataract extraction and intraocular lens implantation. Am J Ophthalmol 114:448, 1992 265. Pollack A, Leiba H, Bukelman A, et al: The course of diabetic retinopathy following cataract surgery in eyes previously
treated by laser photocoagulation. Br J Ophthalmol 76:228, 1992 266. Ulbig MR, Hykin PG, Foss AJ, et al: Anterior hyaloidal fibrovascular proliferation after extracapsular cataract
extraction in diabetic eyes. Am J Ophthalmol 115:321, 1993 267. Schatz H, Atienza D, McDonald HR, et al: Severe diabetic retinopathy after cataract surgery. Am J Ophthalmol 117:314, 1994 268. Hykin PG, Gregson RMC, Hamilton AMP: Extracapsular cataract extraction in diabetics with rubeosis iridis. Eye 6:296, 1992 269. Puvanendran K, Devathasan G, Wong PK: Visual evoked responses in diabetes. J Neurol Neurosurg Psychiatry 46:643, 1983 270. Cirillo D, Gonfiantini E, DeGrandis E, et al: Visual evoked potentials in diabetic children and adolescents. Diabetes Care 7:273, 1983 271. Lubow M, Makely TA: Pseudopapilledema of juvenile diabetes mellitus. Arch Ophthalmol 85:417, 1971 272. Skillern PG, Lockhart G: Optic neuritis and uncontrolled diabetes mellitus in 14 patients. Ann Intern Med 51:468, 1959 273. Yanko L, Ticho U, Ivry M: Optic nerve involvement in diabetes. Acta Ophthalmol 50:556, 1972 274. Regillo CD, Brown GC, Savino PJ, et al: Diabetic papillopathy. Patient characteristics and fundus findings. Arch Ophthalmol 113:889, 1993 275. Appen RE, Chandra SR, Klein R, et al: Diabetic papillopathy. Am J Ophthalmol 90:203, 1980 276. Barr CC, Glaser JS, Blankenship G: Acute disc swelling in juvenile diabetes. Clinical profile and natural history of 12 cases. Arch Ophthalmol 98:2185, 1980 277. Pavan PR, Aiello LM, Wafai Z, et al: Optic disc edema in juvenile-onset diabetes. Arch Ophthalmol 98:2193, 1980 278. Burde RM: Neuro-ophthalmic associations and complications of diabetes mellitus. Am J Ophthalmol 114:498, 1992 |