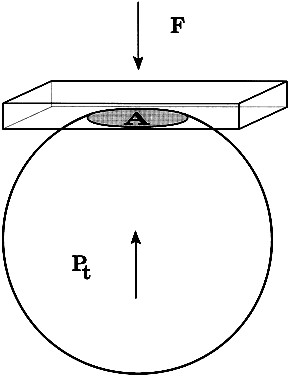
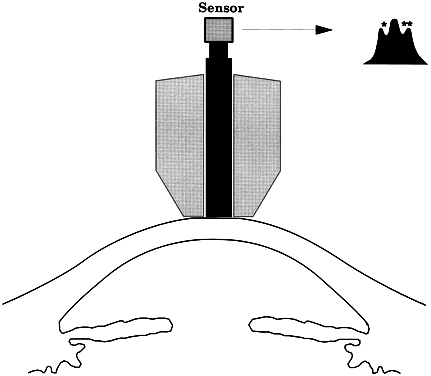
The pressure inside a flexible sphere can be estimated by using fixed forces to create measurable deformations of the wall or, conversely, by using variable forces to produce predetermined deformations of the sphere wall. Both approaches have been used in tonometry of the eye. The methods used are based on the Imbert-Fick principle, which states that if a plane surface is applied to a spherical membrane to cause a flattening of surface area, the pressure inside the spherical membrane will be equal to the applied force divided by the area of contact (Fig. 1).1,2 It must be emphasized that the Imbert-Fick principle is considered valid when the sphere is perfectly round, dry, elastic, and infinitely thin. Because the wall of the eye is none of these, application of the Imbert-Fick principle to tonometry requires careful attention to variations caused by the fundamental nature of the ocular tissues and to careful calibration of all tonometers in the laboratory.
|
During the years 1905 to 1926, Hjalmar August Schiøtz, a Norwegian ophthalmologist, produced and refined a mechanical indentation tonometer that is still in common use.3 This tonometer uses a weighted plunger that rides freely in the center of a hollow cylinder (Fig. 2). The outer cylinder has a footplate with a concave radius of 15-mm curvature, which rests on the cornea during measurement. The weighted plunger indents the cornea centrally, and the amount of indentation is transferred to a scale at the top of the instrument by a lever mechanism, which converts 0.05 mm of plunger movement into 1-mm units on the read-out scale. It is clear that a soft eye allows more plunger indentation than does a hard eye; the Schiøtz scale displays large amounts of indentation as high scale readings and small amounts of indentation as low scale readings.
When the Schiøtz tonometer is placed on the eye, a considerable force is applied to the cornea. This force is a combination of plunger weight plus the weight of the footplate cylinder and the accompanying scale. With the smallest plunger weight (5.5 g), the total weight of the instrument on the eye is 16.5 g. Resting this much weight on the eye raises IOP during the measurement. The pressure of clinical interest is the pressure of the eye in its natural state—that is, before the weight of the tonometer was applied. It is therefore necessary to use calibration tables to translate the readings made during tonometry into an estimate of the pressure that existed in the eye before the application of the tonometer. IOP during tonometry is called Pt, whereas IOP in the resting state before application of the tonometer is known as “P naught” or P0. One of the major contributions of Dr. Jonas Friedenwald was the calibration of the Schiøtz tonometer, which included a careful scientific study of the relation between Pt and P0.4,5
The indentation of the cornea caused by the Schiøtz tonometer displaces a small volume of fluid into the eye. This displaced fluid is accommodated by stretching or expansion of the ocular coats. Dr. Friedenwald found that the volume of fluid displaced was related to the logarithm of Pt by a factor that he called scleral rigidity. The tables that are commonly used to estimate IOP based on a Schiøtz scale reading assume an eye with normal scleral rigidity. It is important to remember that the tables give inaccurate estimates of IOP in eyes that do not have normal scleral rigidity. Perhaps the most common example is the patient with high myopia, a condition commonly associated with low scleral rigidity. In such a case, the eyeball is unusually elastic and allows the weighted plunger to indent the cornea more than an eye of normal scleral rigidity with the same pressure. This gives a falsely high scale reading on the tonometer and a correspondingly falsely low estimate of IOP. Such false low pressures can be hazardous; important elevations of IOP have been missed in myopes and other eyes with low scleral rigidity that were tested by indentation tonometry. This mistake can be avoided by using the applanation type of tonometer or by taking Schiøtz readings with different weights on the plunger and using these values in conjunction with a Friedenwald nomogram to calculate the P0 for that eye.
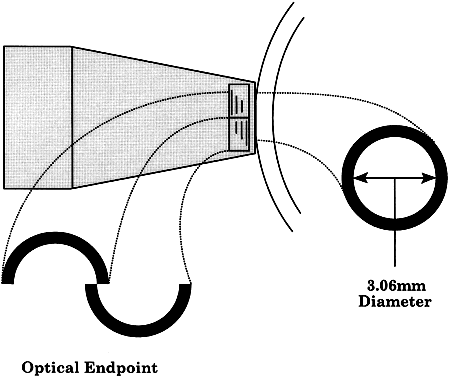
Using an applanation technique in which the cornea is flattened—not indented—eliminates many of the uncertainties of indentation tonometry. In the classic method developed by Goldmann, a circular area of the central cornea is flattened by pushing a plastic tonometer tip against the eye. The circle of applanation is delineated by a fluorescein-stained tear film (Figs. 3 and 4). The force required to flatten a circle of 3.06 mm is in grams a tenth of IOP in mmHg. Therefore, a force of 1.6 g is required to flatten this circular area when IOP is 16 mmHg. The 3.06-mm diameter circle of applanation was chosen because of this simple 10:1 relation between IOP and grams of force; this area is within the range in which the natural bending force of the cornea is canceled by the capillary attraction created by the tear film between the tonometer head and the cornea.6,7 Flattening so small an area of the cornea creates little fluid displacement within the eye. Therefore, scleral rigidity is not a factor in Goldmann applanation tonometry.
|
Although the Goldmann tonometer is generally used at the slit lamp, the principle is not exclusive to that arrangement. Portable tonometers based on this principle (e.g., the Perkins tonometer8 and Draeger tonometer9) allow the increased accuracy of the Goldmann technique to be brought to the bedside, operating room, and glaucoma-screening setting. These portable applanation tonometers are especially useful in young children who may be frightened and restless at the slit lamp.
Other tonometric instruments are available. It is a credit to the design and precision of the Goldmann instrument that virtually all new tonometers are evaluated in comparison with it. The air-puff tonometer (American Optical) works on an applanation principle in which the force required to flatten a portion of the cornea is delivered in a carefully calibrated bolus of air rather than through mechanical contact.10 The moment of flattening is recorded optoelectronically and converted into an estimate of IOP by a computer in the machine. This instrument is especially useful when many patients need to be screened or it is desirable or necessary to avoid topical anesthesia.
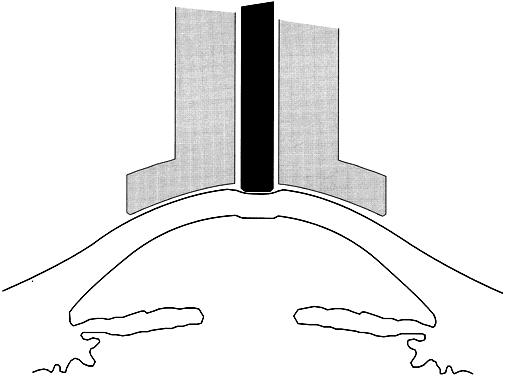
When the cornea is scarred, irregular, or edematous, it is useful to have a tonometer available that does not depend on an optical end point for its measurement. Two instruments are useful in this instance: the Mackay-Marg tonometer11,12 and the Pneumotonometer.13 The Mackay-Marg tonometer has a central piston, with provision to sense force surrounded by a passive annulus (Fig. 5). This instrument is brought up to the cornea by hand and the force necessary to applanate the cornea to the diameter of the central piston is determined by an electronic display.14 In the Pneumatonometer, a plunger is also brought up to the cornea by hand but the actual force of application is supplied by compressed gas. A valving mechanism at the tip of the plunger determines the end point for the measurement; the gas pressure necessary to achieve the end point is sensed and displayed electronically.15 Laboratory and clinical studies have shown that the Pneumatonometer causes some degree of indentation.16,17
An alternative method of applanation tonometry involves the application of fixed forces, with subsequent measurement of the area applanated. This can be done simply with small weights that have a flat surface. The area flattened by a particular weight can be determined either from staining patterns on the face of the weight (Maklakoff instrument); if the applanating surface is transparent, it can be determined from direct visual observation of the flattened area (Halberg instrument).18
NORMAL PRESSURE
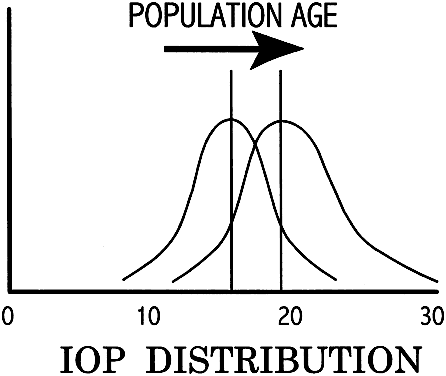
In the normal young adult population, the mean of IOP readings lies between 15 and 16 mmHg, with a distribution that is bell-shaped and symmetric (Fig. 6).19 With increasing age, most studies indicate a tendency toward increased IOP, with skewing of the distribution toward higher pressures.20 Conversely, IOP in normal infants and children tends to run lower than that of the young adult population.21
CIRCADIAN VARIATION IN INTRAOCULAR PRESSURE
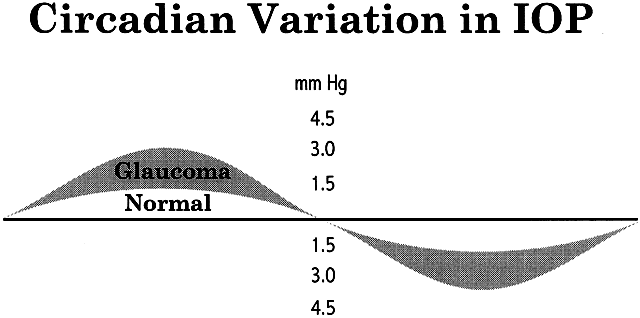
Intraocular pressure shows a natural cycle, with a phase of relative elevation followed by a phase of relative depression occurring over a 24-hour period—a circadian rhythm.22 The mechanism for this variation almost certainly involves variations in aqueous humor production, although other factors contribute also. The magnitude of the variation is greater in glaucoma patients—in some studies, three or four times greater than in the normal population (Fig. 7). The larger pressure swing in glaucoma patients is logically related to poor outflow facility but because outflow facility and diurnal variation of IOP are not highly correlated, other factors also must play a role.
The most common daily cycle shows a tendency for higher pressures in the morning hours and falling pressures in the evening. This pattern is not universal, however, because peak pressures occur at any time of the day. Careful studies of IOP using home tonometry23 to track patients' normal daily patterns have shown that about 50% of peak pressures fall outside normal office hours.24 The practical consequences of circadian variation in IOP are several.
First, no single reading of IOP can be considered to be representative. Likewise, no change in pressure should be labeled a therapeutic success or failure unless diurnal variation has been carefully considered. There are several practical approaches to this problem (Table 1).
TABLE 1. Neutralizing the Effect of Circadian IOP Variation
Record the time of all pressure readings
When changing therapy, schedule the next appointment at the same time of
day
When not changing therapy, vary the time of appointments to help reveal
the circadian pattern
If a consistent “peak time” is found, most appointments should
be scheduled at or near this time
When a patient seems to be losing ground despite “good IOPs,” schedule
a series of pressure readings throughout the longest practical
period
If practical, instruct a family member in home tonometry
IOP = intraocular pressure.
POTENTIAL ERRORS IN TONOMETRY
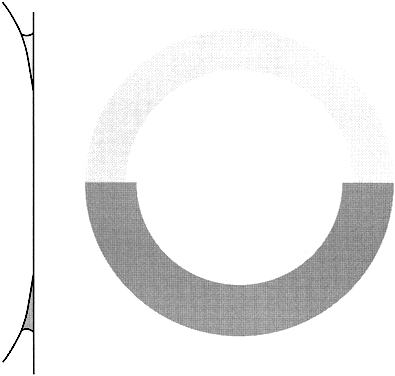
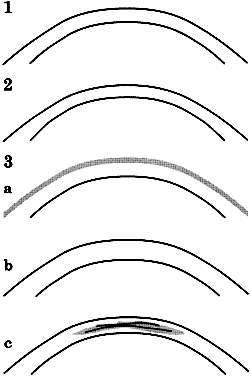
As noted, the eye does not meet the ideal criteria of the Imbert-Fick principle. This is particularly crucial at the cornea-tonometer interface, where variations in corneal thickness, flexibility, or composition can introduce substantial errors (Fig. 8). Abnormal thickness produces false high readings,25,26 whereas corneas that are thinner than normal—either naturally27 or as the result of laser refractive surgery28,29—produce false low readings. Numerous other factors have potential to produce important errors; many are listed in Table 2 and an extensive review is available. Some errors of tonometry can only be detected reliably by in vivo comparison of tonometric measurements with simultaneous manometry (see Table 2).16,30
TABLE 2. Sources of Error in Tonometry
Ocular/Periocular Anomalies
- Lid, muscle, orbit malformation, infiltration, or congestion
- Corneal anomalies: thickness, scarring, edema (see Fig. 8)
- Absence of “aqueous free space” behind cornea48
- Abnormal scleral rigidity (indentation tonometry)
Patient-induced
- Lid squeezing
- Breathholding, constrictive clothing
- Eye/head movement
- Unsuspected/unreported drug effects (usually → lower IOP) (e.g., recent
ethanol ingestion, marijuana, systemic beta blockers)
- Recent exercise (usually → lower IOP,49 may → higher IOP in pigmentary glaucoma)
- Extraneous forces: hair, 50 moustache51
Instrument Error
- Poor maintenance, cleaning
- Out of calibration
Operation Error
- Failure to consider/observe any of the above
- Applying pressure to the lids
- Using inappropriate fluorescein concentration
- Failure to establish steady state through patient observation, repeat measurement
- Failure to record time of day
IOP = intraocular pressure.
STERILIZATION OF TONOMETERS
Because tears may contain pathogens capable of causing both serious ocular disease and potentially fatal systemic disease, it is essential to disinfect all tonometers between patients. The MacKay-Marg and Tonopen require a protective cover. If this is changed between patients, no further sterilization is necessary. Although protective covers are available for other types of tonometers, chemical cleaning is used more often. Soaking the tonometer tip in a special container holding dilute (1:10) household bleach, hydrogen peroxide in a 3% solution, or 70% isopropyl alcohol is highly effective but great care must be taken to remove these toxic substances before contacting the eye. For practical purposes, many centers accept careful wiping with an alcohol sponge for tonometer disinfection.31
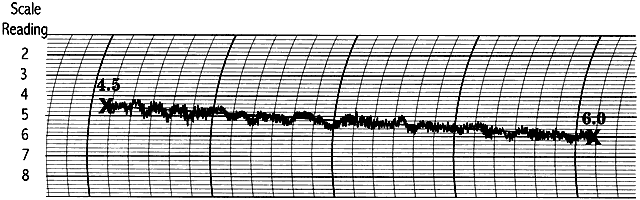
 Where ΔV
is the volume change in μL and T is time in minutes—usually 4. Substituting, for this case,
Where ΔV
is the volume change in μL and T is time in minutes—usually 4. Substituting, for this case,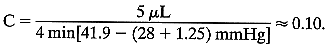 As here, 1.25 is usually added to F0 to compensate for the small increase in episcleral venous pressure induced
by tonography. The P0/C ratio for this eye is 28/0.10, or 280. Stated another way, under the
influence of an average increased IOP of 12.65 mmHg (induced by applying
a Schiøtz tonometer with a 7.5-g weight), an extra 5 μL
of aqueous humor was forced through the outflow channels during a 4-minute
period. This reflects a low aqueous outflow.
As here, 1.25 is usually added to F0 to compensate for the small increase in episcleral venous pressure induced
by tonography. The P0/C ratio for this eye is 28/0.10, or 280. Stated another way, under the
influence of an average increased IOP of 12.65 mmHg (induced by applying
a Schiøtz tonometer with a 7.5-g weight), an extra 5 μL
of aqueous humor was forced through the outflow channels during a 4-minute
period. This reflects a low aqueous outflow.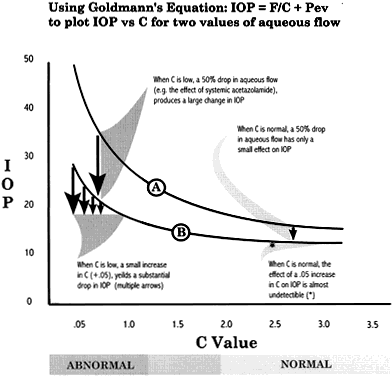
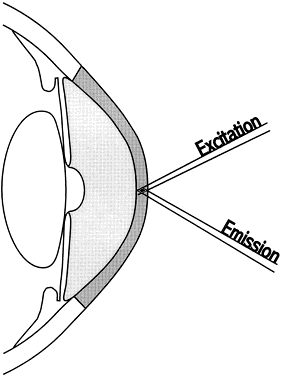
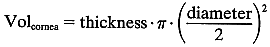 In this case:
In this case: 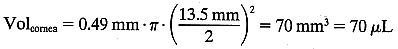 The volume of the anterior chamber is estimated using the formula for a segment of a sphere:
The volume of the anterior chamber is estimated using the formula for a segment of a sphere: 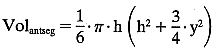 where h = axial depth of the anterior chamber and y = anterior chamber
diameter. In this case:
where h = axial depth of the anterior chamber and y = anterior chamber
diameter. In this case: 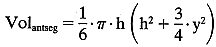 The total mass of fluorescein in the cornea and the anterior segment can
then be calculated:
The total mass of fluorescein in the cornea and the anterior segment can
then be calculated: 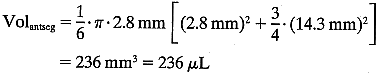 The substraction of 1.5 ng/ml corrects for corneal autofluorescence in
this patient.
The substraction of 1.5 ng/ml corrects for corneal autofluorescence in
this patient. 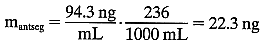 So, at 0800 hrs,
So, at 0800 hrs, 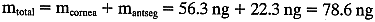 By similar means, the mass at 0900 hours is calculated to be 60.6 ng, so
the loss of fluorescein over the 1-hour period = 18 ng. As a first
approximation, it is assumed that this 18 ng/60 minutes loss occurs completely
from aqueous flow. Calculating average aqueous concentration
during the hour:
By similar means, the mass at 0900 hours is calculated to be 60.6 ng, so
the loss of fluorescein over the 1-hour period = 18 ng. As a first
approximation, it is assumed that this 18 ng/60 minutes loss occurs completely
from aqueous flow. Calculating average aqueous concentration
during the hour: 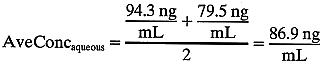 So, 1000 μL of aqueous humor “carries” 86.9 ng of fluorescein. We
can then derive the flow rate necessary to remove this mass
of fluorescein:
So, 1000 μL of aqueous humor “carries” 86.9 ng of fluorescein. We
can then derive the flow rate necessary to remove this mass
of fluorescein: 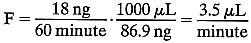 In fact, because a small amount (5% to 10%) of the fluorescein leaves
the anterior segment by diffusion into the iris, the true aqueous flow
rate is correspondingly less. In practice, the test is more complicated
than this example suggests. For one thing, the readings are usually
repeated over several hours, and the necessary measurements and calculations
are often more complex than this example. More detailed discussions
are available.
In fact, because a small amount (5% to 10%) of the fluorescein leaves
the anterior segment by diffusion into the iris, the true aqueous flow
rate is correspondingly less. In practice, the test is more complicated
than this example suggests. For one thing, the readings are usually
repeated over several hours, and the necessary measurements and calculations
are often more complex than this example. More detailed discussions
are available.