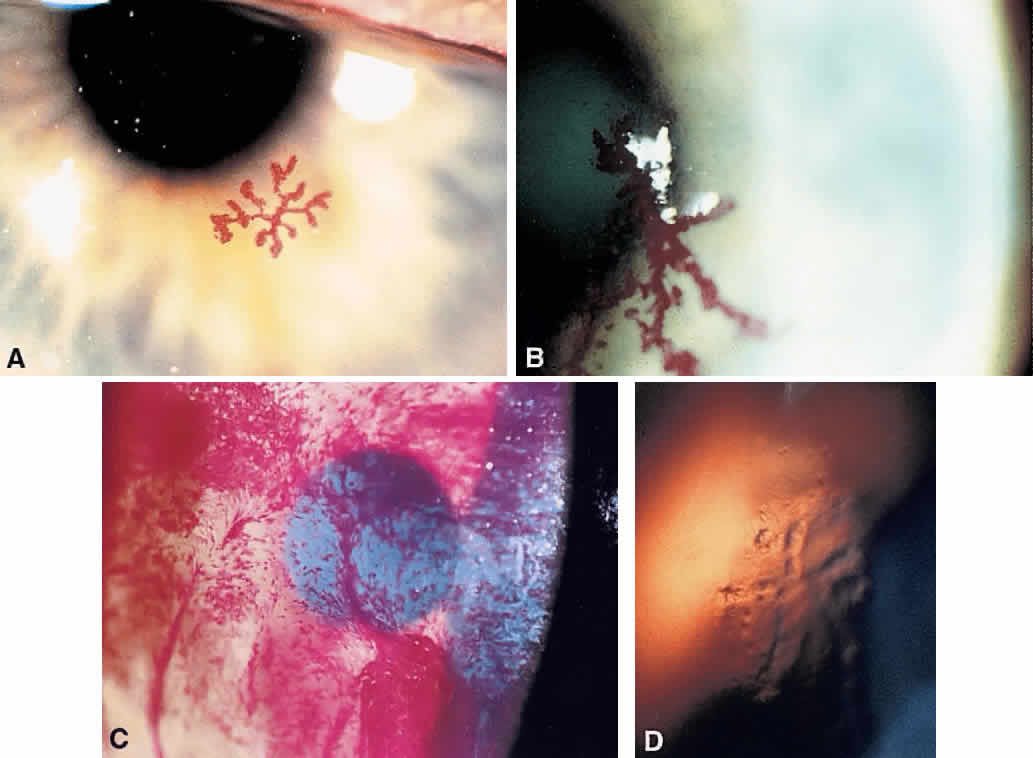
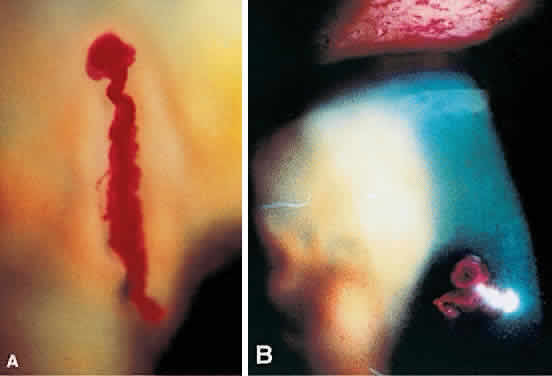
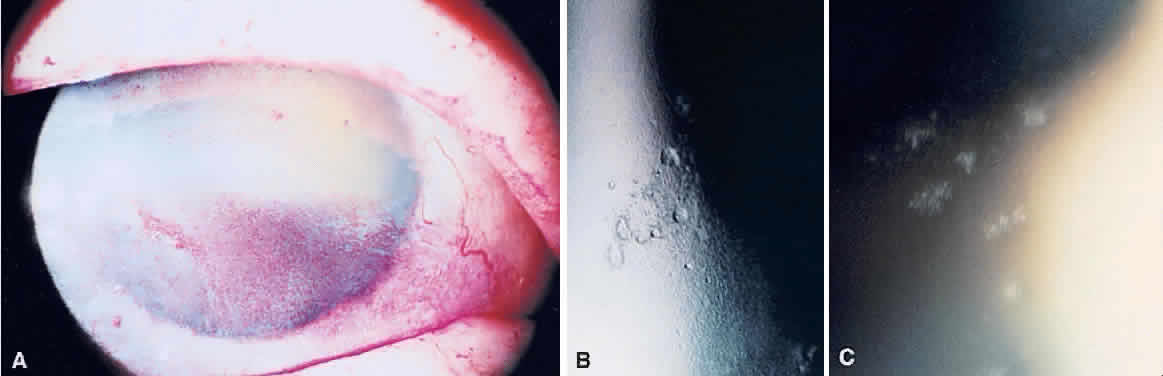
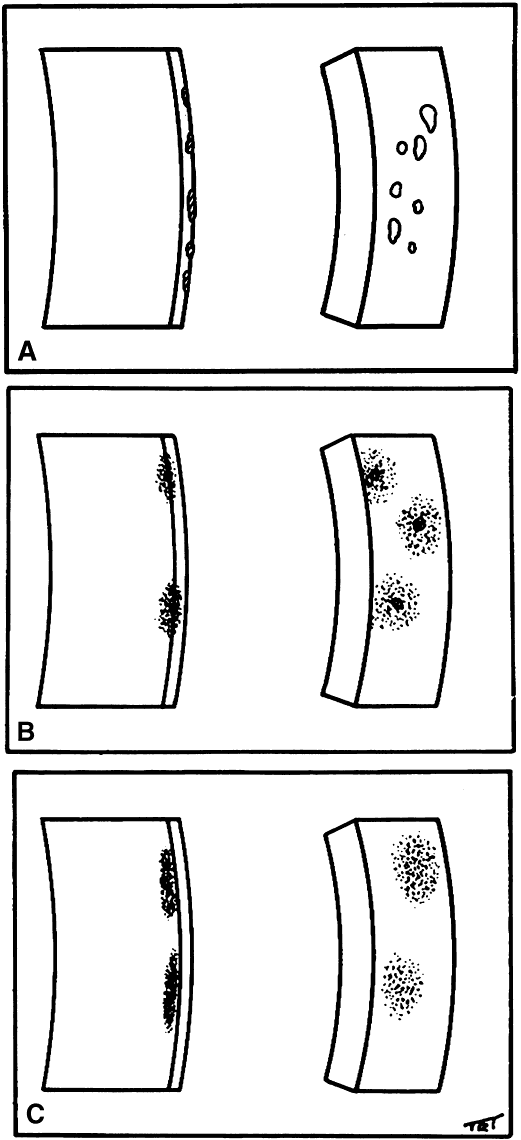
1. Norn MS: Rose bengal vital staining. Acta Ophthalmol 48:546, 1970 2. Coster DJ, Grutzmacher RD: Herpetic eye disease. Aust J Ophthalmol 11:1, 1983 3. Marsh RJ, Fraunfelder FT, McGill JI et al: Herpetic corneal epithelial disease. Arch Ophthalmol 94:1899, 1976 4. Dawson CR, Togni B: Herpes simplex eye infectons: Clinical manifestations, pathogenesis and
management. Surv Ophthalmol 21:121, 1976 5. Piebenga LW, Laibson PR: Dendritic lesions in herpes zoster ophthalmicus. Arch Ophthalmol 90:268, 1973 6. Marsh RJ: Herpes zoster keratitis. Trans Ophthalmol Soc UK 93:181, 1973 7. Acers TE, Vaile V: Co-existent herpes zoster and herpes simplex. Am J Ophthalmol 63:992, 1967 8. Chern KC, Conrad D, Holland GN et al: Chronic varicella-zoster virus epithelial keratitis in patients with acquired
immunodeficiency syndrome. Arch Ophthalmol 116:1011, 1998 9. Beinfang DC, Kuwabara T, Pueschel SM: The Richner-Hanhart syndrome. Arch Ophthalmol 94:1133, 1976 10. Goldsmith LA, Reed J: Tyrosine-induced eye and skin lesions. A treatable genetic disease. JAMA 236:382, 1976 11. Burns RP: Soluble tyrosine aminotransferase deficiency: An unusual case of corneal
ulcers. Am J Ophthalmol 73:400, 1972 12. Abdel-Khalek LMR, Williamson J, Lee WR: Morphological changes in the human conjunctival epithelium, II. In keratoconjunctivitis
sicca. Br J Ophthalmol 62:800, 1978 13. Wright P: Sjögren's syndrome. Trans Ophthalmol Soc UK 94:764, 1974 14. Fraunfelder FT, Wright P, Tripathi RD: Corneal mucous plaques. Am J Ophthalmol 83:191, 1977 15. Jones BR: Vernal keratitis. Trans Ophthalmol Soc UK 81:215, 1981 16. Williamson J, Doig WM, Forrester JV et al: Management of the dry eye in Sjögren's syndrome. Br J Ophthalmol 58:798, 1974 17. Wright P: Diagnosis and management of dry eyes. Trans Ophthalmol Soc UK 91:119, 1971 18. Theodore FH: Superior limbic keratoconjunctivitis. Eye Ear Nose Throat Monthly 42:25, 1963 19. Thygeson P: Further observations on superficial punctate keratitis. Arch Ophthalmol 66:34, 1961 20. Theodore FH: Further obsrvations on superior limbic keratoconjunctivitis. Trans Am Acad Ophthalmol Otolaryngol 71:341, 1967 21. Theodore FH, Fury AP: Superior limbic keratoconjunctivitis: clinical and pathological correlations. Arch Ophthalmol 84:481, 1970 22. Wright P: Superior limbic keratoconjunctivitis. Trans Ophthalmol Soc UK 92:555, 1972 23. Dodds HT, Laibson PR: Filamentary keratitis following cataract extraction. Arch Ophthalmol 88:609, 1972 24. Tenzel RR: Comments on superior limbic filamentous keratitis, Part 2. Arch Ophthalmol 79:508, 1968 25. Cher I: Clinical features of superior limbic keratoconjunctivitis in Australia: A
probable association with thyrotoxicosis. Arch Ophthalmol 82:580, 1969 26. Fells P: Comments on superior limbic keratoconjunctivitis. Trans Ophthalmol Soc UK 92:50, 1972 27. Bloomfield SE, Gasset AR, Forstot SL et al: Treatment of keratitis with soft contact lens. Am J Ophthalmol 76:978, 1973 28. Tenzel RR: Resistant superior limbic keratoconjunctivitis. Arch Ophthalmol 89:439, 1973 29. Donshik PC, Collin HB, Foster CS et al: Conjunctival resection treatment and ultrastructural histopathology of
superior limbic keratoconjunctivitis. Am J Ophthalmol 85:101, 1978 30. Rothis WM, Chandler JW, Forstot SL: Filamentary keratitis following penetrating keratoplasty. Ophthalmology 89:946, 1982 31. Davis WG, Drewsy RD, Wood TO: Filamentary keratitis and stromal neovascularization associated with brain
stem injury. Am J Ophthalmol 90:489, 1980 32. Wolper J, Laibson PR: Hereditary hemorrhagic telangiectasis (Rendu-Osler-Weber disease) with
filamentary keratitis. Arch Ophthalmol 81:272, 1969 33. Hudson AC: A note on certain peculiar markings in the cornea. R London Ophthalmic Hosp Rep 18:198, 1911 34. Stähli J: Uber den Fleischerschen Ring beim Keratokonus und eine neue typische Epithelpigmentation
der mornalen Kornea. Klin Monatsbl Augenheilkd 60:721, 1918 35. Barraguer-Somers E, Chan CC, Green WR: Corneal epithelial iron deposition. Am J Ophthalmol 90:729, 1983 36. Stocker F: Eine pigmentierte Hornhautlinie beim Pterygium. Klin Monatsbl Aukenheilkd 102:384, 1939 37. Ferry AP: A “new” iron line of the superficial cornea: Occurrence in
patients with filtering blebs. Arch Ophthalmol 79:142, 1968 38. Fleischer B: Uber Keratokonus und elgenartige Pigmentbildung in der Kornea. MMWR 53:625, 1906 39. Bron AJ: Vortex patterns of the corneal epithelium. Trans Ophthalmol Soc UK 93:455, 1973 40. Hobbs HE, Calnan CD: The ocular complications of chloroquine therapy. Lancet 1:1207, 1958 41. Kaplan LJ, Cappaert WE: Amiodarone keratopathy. Arch Ophthalmol 100:601, 1982 42. Kersley GD, Palin AG: Amodioquine and hydroxychloroquine in rheumatoid arthritis. Lencet 2:886, 1959 43. Burns CA: Indomethacin, reduced retinal sensitivity and corneal deposits. Am J Ophthalmol 66:825, 1968 44. Francois J: Cornea verticillata. Bull Soc Belge Ophthalmol 150:656, 1968 45. Lullmann H, Lullmann-Rauch R, Wassermann O: Lipidosis induced by amphiphilic cationic drugs. Biochem Pharmacol 27:1103, 1978 46. Font RL, Fine BS: Ocular pathology in Fabry's disease. Am J Ophthlmol 73:419, 1972 47. Cowan TH: Striate melanokeratosis in Negroes. Am J Ophthalmol 57:443, 1964 48. Jones BR: The differential diagnosis of punctate keratitis. Trans Ophthalmol Soc UK 80:655, 1961 49. Fuchs E: Keratitis punctate superficial. Wien Klin Wochenschr 2:837, 1889 50. Sanders M: Epidemic keratoconjunctivities (“shipyard conjunctivitis”), Part
I. Isolation of a virus. Arch Ophthalmol 28:581, 1942 51. Braley AE, Alexander RC: Superficial punctate keratitis. Arch Ophthalmol 50:147, 1953 52. Burstein NL: Preservative cytotoxic threshold for bensalkonium chloride and chlorhexidine
digluconide in cat and rabbit corneas. Invest Ophthalmol Vis Sci 19:308, 1980 53. Schwab IR, Abbott RL: Toxic ulcerative keratopathy. Ophthalmology 96:1187, 1989 54. Abbott RL, Forster RK: Superficial punctate keratitis of Thygeson associated with scarring and
Salzmann's nodular degeneration. Am J Ophthalmol 87:296, 1979 55. Thygeson P: Superficial punctate keratitis. JAMA 144:1544, 1950 |