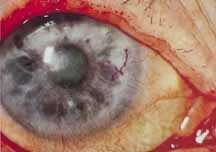
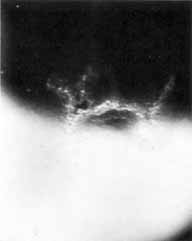
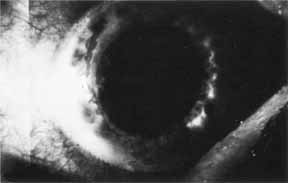
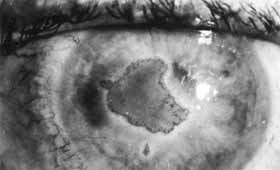
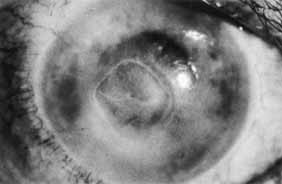
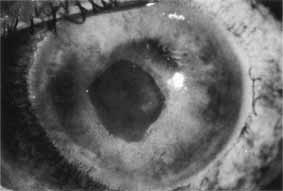
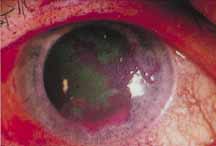
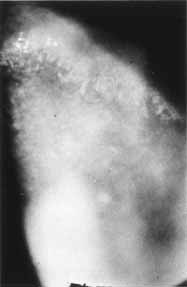
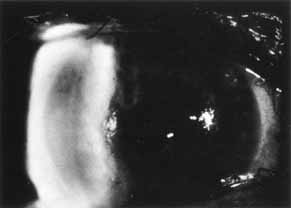
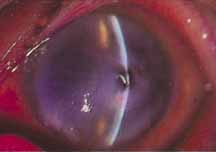
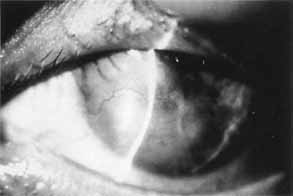
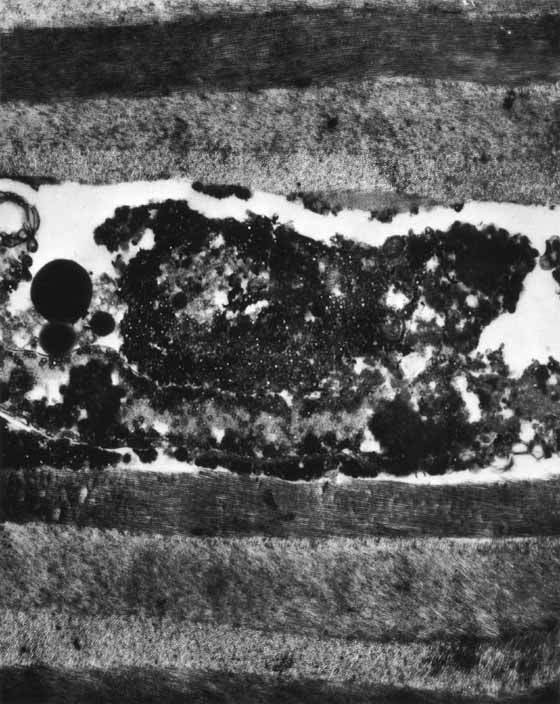
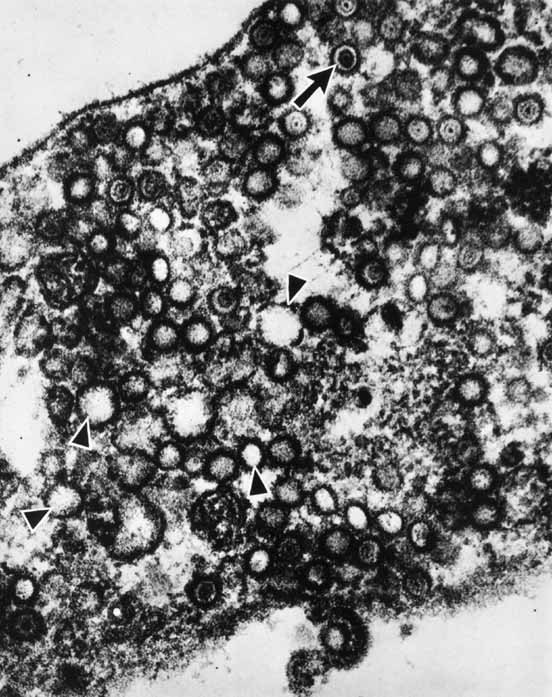
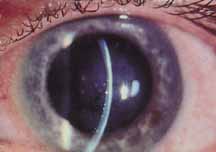
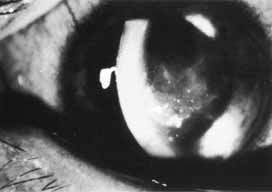
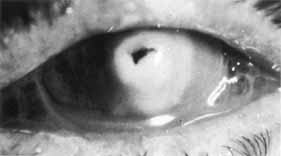
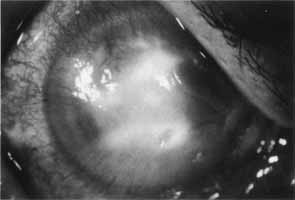
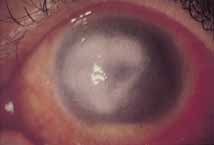
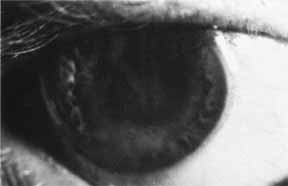
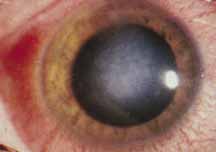
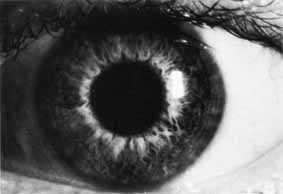
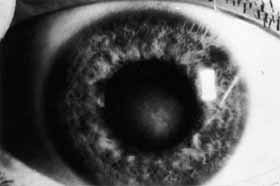
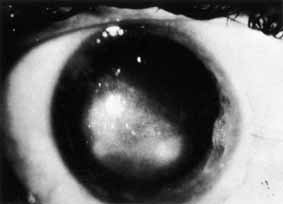
1. Brik D, Dunkel E, Pavan-Langston D: Herpetic keratitis: Persistence of viral particles despite topical and
systemic antiviral therapy. Report of two cases and review of the literature. Arch Ophthalmol 111:522, 1993 2. Mindel A: Epidemiology. In Herpes Simplex Virus. New York: Springer-Verlag, 1989:33 3. Liesegang TJ: Biology and molecular aspects of herpes simplex and varicella-zoster
virus infections. Ophthalmology 99:781, 1992 4. Corey L, Spear PG: Infections with herpes simplex viruses. N Engl J Med 314:686, 1986 5. Cook SD: Herpes simplex virus in the eye. Br J Ophthalmol 76:365, 1992 6. Shieh MT, Spear PG: Herpesvirus-induced fusion that is independent on cell surfaced
heparin sulphate or soluble heparin. J Virol 68:1224, 1994 7. Darlington RW, Moss LH: III: Herpesvirus envelopment. J Virol 2:48, 1968 8. Rosenwasser GO, Greene WH: Simultaneous herpes simplex types 1 and 2 keratitis in acquired immunodeficiency
syndrome. Am J Ophthalmol 113:102, 1992 9. Kibrick S, Goodling GW: Pathogenesis of infection with herpes simplex virus with special reference
to nervous tissue in slow, latent, and temporate virus infections. NINDB Monogr 2:143, 1965 10. Nahmias et al: Sero-epidemiological and sociological patterns of herpes simplex
virus infections in the world. Scand J Infect Dis 69(supp) :19, 1990 11. Kaufman HE, Brown DC, Ellison ED: Herpesvirus in the lacrimal gland, conjunctiva and corneal of man—a
chronic infection. Am J Ophthalmol 65:32, 1968 12. Kaufman HE: In vivo studies with antiviral agents. Ann N Y Acad Sci. 130:168, 1965 13. Lindgren KM, Douglas GR Jr, Couch RB: Significance of Herpesvirus hominis in respiratory secretions of man. N Engl J Med 278:517, 1968 14. Rawls WE, Campione-Picardo J: Epidemiology of herpes simplex virus type 1 and type 2 infections. In Nahmias AJ, Dowdle WR, Schinazi RF, eds: The Human Herpesviruses. An Interdisciplinary Perspective. New York: Elsevier, 1981:139 15. Arffa RC: Viral diseases. In Grayson's Diseases of the Cornea, 3rd ed. St. Louis: CV Mosby, 1991:238–294 16. Dhaliwal DK, Romanowski EG, Yates KA, et al: Experimental laser-assisted in situ keratomileusis induces the reactivation
of latent herpes simplex virus. Am J Ophthalmol 131:506, 2001 17. Perry HD, Doshi SJ, Donnenfelf ED, et al: Herpes simplex reactivation following laser in situ keratomileusis and
subsequent corneal perforation. CLAO J 28:69, 2002 18. Liesegang TJ, Melton J, Daly PF, et al: Epidemiology of ocular herpes simplex.Incidence in Rochester, MN, 1950 through 1982. Arch Ophthalmol 107:1155, 1989 19. Gordon YJ: Pathogenesis and latency of herpes simplex virus type 1 (HSV-1): an
ophthalmologist's view of the eye as a model for the
study of the virus-host relationship. Adv Exp Med Biol 278:205, 1990 20. Goodpasture EW: Herpetic infection with special reference to involvement of the nervous
system. Medicine 8:223, 1929 21. Roizman B: In Pollard M (ed): Perspectives in Virology. New York: Harper & Row, 1965 22. Fenner F: The Biology of Animal Viruses. New York: Academic Press, 1968 23. Stevens JG, Cook ML: Latent herpes simplex virus in spinal ganglia of mice. Science 73:740, 1971 24. Cook ML, Stevens JG: Pathogenesis of herpetic neuritis and gangliomites in mice: evidence of
intra-axonal transport of infection. Infect Immunol 7:272–288, 1973 25. Pepose JS, Lieb DA, Stuart M, Easty D: Ocular infections and immunity. In Herpes Simplex Virus Diseases: Anterior Segment of the Eye. St. Louis: Mosby, 1995:908–909 26. Fraser NW, Spivack JG, Wroblewska Z, et al: A review of the molecular mechanism of HSV-1 latency. Curr Eye Res 9:1, 1990 27. Cook D, Hill JH: Herpes simplex virus: molecular biology and the possibility of corneal
latency. Surv Ophthalmol 36:140, 1991 28. Rong BL, Kenyon KR, Bean KM, et al: Detection of the herpes simplex virus genome in the human cornea. Ophthalmology 95(suppl):159, 1988 29. Abghare SZ, Stulting RD: Recovery of herpes simplex virus from ocular tissues of latently infected
inbred mice. Invest Ophthalmol Vis Sci 29:239, 1988 30. Cook SD, Batra SK, Brown SM: Recovery of herpes simplex virus from corneas of experimentally infected
rabbits. J Gen Virol 68:2013, 1987 31. Gerdes JC, Smith DS: Recurrence phenotypes and establishment of latency following rabbit keratitis
produced by multiple herpes simplex virus strains. J Gen Virol 64:2441, 1983 32. Centifanto-Fitzgerald YM, Fenger T, Kaufman HE: Virus proteins in herpetic keratitis. Exp Eye Res 35:425, 1982 33. Smeraglia R, Varnell ED, Centifanto UM, et al: The role of herpes simplex virus secreted glycoproteins in herpetic keratitis. Exp Eye Res 35:443, 1982 34. Kaufman HE, Varnell ED, Centifanto YM, et al: Effect of the herpes simplex virus genome on the response of infection
to corticosteroids. Am J Ophthalmol 100:114, 1985 35. Jones BR: The management of ocular herpes. Trans Ophthalmol Soc UK 79:425, 1959 36. Coster DJ, Jones BR, Falcon MG: Role of debridement in the treatment of herpetic keratitis. Trans Ophthalmol Soc UK 97:314, 1977 37. Pavan-Langston D, Dohlman CH, Geary P, et al: Intraocular penetration of AraA and IDU—Therapeutic implication in
clinical herpetic uveitis. Trans Am Acad Ophthalmol Otolaryngol 77:455, 1973 38. Laibson PR, Kratchmer JH: Controlled comparison of adenine arabinoside and idoxuridine therapy of
human superficial dendritic keratitis. In Pavan-Langston D, Buchanan RA, Alford GA, eds. Adenine Arabinoside: An Anti-Viral Agent. New York: Raven Press, 1976:323 39. Teich SA, Cheung TW, Friedman AH: Systemic antiviral drugs used in ophthalmology. Surv Ophthalmol 37:19, 1992 40. LaLau C, Oosterhuis JA, Versteeg J, et al: Acyclovir and trifluorothymidine in herpetic keratitis—a multicenter
trial. Br J Ophthalmol 66:506, 1982 41. LaLau C, Oosterhuis JA, Versteeg J, et al: Multicenter trial of acyclovir and trifluorothymidine in herpetic keratitis. Am J Med 73:305, 1982 42. Bialasiewicz AA, Jahn GJ: Systemische acyclovir—Therapie bei rezi divierender durch herpes
simplex virus bedingter keratouveitis. Klin Monatsbl Augenheilkd 185:539, 1984 43. Collum LMT, Akhtar J, McGettrick P: Oral acyclovir in herpetic keratitis. Trans Ophthalmol Soc UK 104:629, 1985 44. Collum LMT, McGettrick P, Akhtar J, et al: Oral acyclovir (Zovirax) in herpes simplex dendritic corneal
ulceration. Br J Ophthalmol 70:435, 1986 45. Wilhelmus KR: The treatment of herpes simplex virus epithelial keratitis. Trans Am Ophthalmol Soc 98:505, 2000 46. Beutner KR: Valcyclovir: a review of its antiviral activity, pharmacokinetic properties
and clinical efficacy. Antiviral Res 28:281, 1995 47. Bohigan G, Dawson C, Coleman V: Retrobulbar administration of steroids in herpes simplex uveitis. Arch Ophthalmol 85:320, 1971 48. Liebowitz HM, Frangie JP: Inflammation of the cornea and its management. In Leibowitz H, Waring GO, eds. Corneal Disorders: Clinical Diagnosis and Management. 2nd ed. Philadelphia: WB Saunders, 1998:529–530 49. Margolis TP, Ostler HB: Treatment of ocular disease in eczema herpeticum. Am J Ophthalmol 110:274, 1990 50. Tullo AB, Easty DL, Shimeld C, et al: Isolation of herpes simplex virus from corneal discs of patients with chronic
stromal keratitis. Trans Ophthalmol Soc UK 104:159, 1985 51. Dresner AJ, Seamans ML: Evidence of the safety and efficacy of adenine arabinoside in the treatment
of herpes simplex epithelial keratitis. In Pavan-Langston D, Buchanan RA, Alford CA Jr, eds. Adenine Arabinoside: An Antiviral Agent. New York: Raven Press, 1975:382 52. Norn MS: Dendritic (herpetic) keratitis: IV. Follow-up examination
of corneal sensitivity. Acta Ophthalmol 48:383, 1970 53. Beiji B, Algawi K, Foley-Nolan A, et al: Herpes simplex in children. Br J Ophthalmol 78:458, 1994 54. Hogan MJ, Kimura SJ, Thygeson P: Pathology of herpes simplex kerato-iritis. Trans Am Ophthalmol Soc 61:75, 1963 55. Dawson C, Togni B, Moore TE: Structural changes in chronic herpetic keratitis studied by light electron
microscopy. Arch Ophthalmol 79:740, 1968 56. Easty DL, Shimeld C, Claove CMP, et al: Herpes simplex virus isolation in chronic stromal keratitis: Human and
laboratory studies. Curr Eye Res 6:69, 1987 57. Font RL: Chronic ulcerative keratitis caused by herpes simplex virus. Electron microscopic confirmation in paraffin-embedded tissue. Arch
Ophthalmol 90:382, 1973 58. Pepose JS: Herpes simplex keratitis: Role of viral infection versus immune response. Surv Ophthalmol 35:345, 1991 59. Pavan-Langston D: Herpetic infections. In Smolin G, Thoft RA, eds. The Cornea. Scientific Foundation and Clinical Practice, 3rd ed. Boston: Little, Brown & Co, 1994:169–215 60. Meyers R: Immunology of herpes simplex virus infection. Int Ophthalmol Clin 15:37, 1975 61. Meyers-Elliott R, Elliott JH, Maxwell WA, et al: HLA antigens in herpes stromal keratitis. Am J Ophthalmol 89:54, 1980 62. Meyers-Elliott R, Pettit T, Maxwell W: Viral antigens in the immune rings of herpes simplex stromal keratitis. Arch Ophthalmol 98:897, 1980 63. Wilhelmus KR, Coster DJ, Donovan HC, et al: Prognostic indicators of herpetic keratitis. Analysis of a five-year
observation period after corneal ulceration. Arch Ophthalmol 99:1578, 1981 64. Holbach LM, Font RL, Baehr W, et al: HSV antigens and HSV DNA in avascular and vascularized lesions of human
herpes simplex keratitis. Curr Eye Res 10(suppl):63, 1991 65. Holbach L, Font R, Naumann G: Herpes simplex stromal and endothelial keratitis. Ophthalmology 97:722, 1990 66. Wilhelmus KR, Falcon MG, Jones BR: Bilateral herpetic keratitis. Br J Ophthalmol 65:385, 1981 67. Dunkel EC, Pavan-Langston D, Fitzpatrick K, et al: Rapid detection of herpes simplex virus (HSV) antigen in human
ocular infections. Curr Eye Res 7:661, 1988 68. Gebhardt BM, Reidy J, Kaufman HE: An affinity membrane test for superficial corneal herpes. Am J Ophthalmol 105:686, 1988 69. Dawson CR, Jones DB, Kaufman HE, et al: Design and organization of the herpetic eye disease study (HEDS). Curr Eye Res 10:105, 1991 70. Dawson CR: The Herpetic Eye Disease Study. Arch Ophthalmol 108:191, 1990 71. Pavan-Langston D, Abelson MB: The role of steroids in ocular herpes. In Boruchoff SA, Hutchinson BR, Lessell S, eds. Controversies in Ophthalmology. Philadelphia: WB Saunders, 1970:438 72. Pavan-Langston D, Abelson MB: Glucocorticoid therapy in ocular herpes simplex. II. Advantages. Surv Ophthalmol 23:43, 1978 73. Ostler HB: Glucocorticoid therapy in ocular herpes simplex. I. Limitations. Surv Ophthalmol 23:35, 1978 74. Thygeson P: The unfavorable role of corticosteroids in herpetic keratitis. In Boruchoff SA, Hutchinson BR, Lessell S, eds. Controversies in Ophthalmology. Philadelphia: WB Saunders, 1970:450 75. Kibrick S, Takahashi GH, Leibowitz HM, et al: Local corticosteroid therapy and reactivation of herpetic keratitis. Arch Ophthalmol 86:694, 1971 76. Easterbrook M, Wilkie J, Coleman V, et al: The effect of topical corticosteroid on the susceptibility of immune animals
to reinoculation with herpes simplex. Invest Ophthalmol Vis Sci 2:181, 1973 77. Wilhelmus KR, Gee L, Hauck WW, et al: The Herpetic eye disease study. A controlled trial of topical corticosteroids
for herpes simplex keratitis. Ophthalmology 101:1883, 1994 78. Wilhelmus KR: Diagnosis and management of herpes simplex stromal keratitis. Cornea 6:286, 1987 79. Kaufman HE, Martola EL, Dohlman C: Use of 5–iodo–2'deoxyuridine (IDU) in treatment
of herpes simplex keratitis. Arch Ophthalmol 68:235, 1962 80. Pavan-Langston D, Nelson DJ: Intraocular penetration of trifluridine. Am J Ophthalmol 87:814, 1979 81. Collum LMT, Logan P, Ravenscroft T: Acyclovir (Zovirax) in herpetic disciform keratitis. Br J Ophthalmol 67:115, 1983 82. VanGanswijk R, Oosterhuis JA, Swart-Van Den Berg M, et al: Acyclovir treatment in stromal herpetic keratitis. Doc Ophthalmol 55:57, 1983 83. Colin J, Malet F, Chastel C, et al: Acyclovir in herpetic anterior uveitis. Ann Ophthalmol 23:28, 1991 84. Porter SM, Patterson A, Kho P: A comparison of local and systemic acyclovir in the management of herpetic
disciform keratitis. Br J Ophthalmol 74:283, 1990 85. Schwab IR: Oral acyclovir in the management of herpes simplex ocular infections. Ophthalmology 95:423, 1988 86. Sundmacher R: Oral acyclovir—Therapie virologisch nachgewiesener intraokularer
herpes simplex virus infektionen. Klin Monatsbl Augenheilkd 183:246, 1983 87. Sanitato JJ, Asbell PA, Varnell ED, et al: Acyclovir in the treatment of herpetic stromal disease. Am J Ophthalmol 98:537, 1984 88. Barron BA, Gee L, Hauck WW, Kurinji N, et al: Herpetic eye disease study. A controlled trial of oral acyclovir for herpes
simplex stromal keratitis. Ophthalmology 101:1871, 1994 89. Lass J, Pavan-Langston D, Berman M: Treatment of experimental herpetic interstitial keratitis with medroxyprogesterone. Arch Ophthalmol 98:520, 1980 90. Gordon YJ: Herpetic stromal keratitis: A new molecular model and its clinical correlation. In Cavanagh HD, ed. The Cornea. Transactions of the World Congress on the Cornea III. New York: Raven Press, 1988:425 91. Metcalf JF, Reichert RW: Histological and electron microscopic studies of experimental herpetic
keratitis in the rabbit. Invest Ophthalmol Vis Sci 18:1123, 1979 92. Oh JO: Endothelial lesions of rabbit cornea produced by herpes simplex virus. Invest Ophthalmol 9:196, 1970 93. Sundmacher R, Neumann-Haefelin D: Herpes simplex virus-positive and virus-negative keratouveitis. In Silverman AM, O'Connor GR, eds. Immunology and Immunopathology of the Eye. New York: Masson, 1979:225 94. Vannas A, Ahoner R, Makitie J: Corneal endothelium in herpetic keratouveitis. Arch Ophthalmol 101:913, 1983 95. Sundmacher R, Neumann-Haefelin D: Herpes simplex virus-positive and negative keratouveitis. In Silverstein AM, O'Connor R, eds. Immunology and Immunopathology of the Eye. New York: Masson, 1979:225–229 96. Power WJ, Hillery MP, Benedict-Smith A, et al: Acyclovir ointment plus topical betamethasone or placebo in first episode
disciform keratitis. Br J Ophthalmol 76:711, 1992 97. Olsen TW, Hardten DR, Meiusi RD, et al: Linear endotheliitis. Am J Ophthalmol 117:468, 1994 98. Vogel A, Schneider H, Loffler KU: Histopathology of herpetic corneal endotheliitis. Klinische Monatsblatter for Augenheilkunde 219:449, 2002 99. Holland EJ, Schwartz GS: Classification of herpes simplex keratitis. Cornea 18: 144, 1999 100. Brown DD, McCulley JP, Bowman RW, et al: The use of conjunctival flaps in the treatment of herpes keratouveitis. Cornea 11:44, 1992 101. Lesher MP, Lohman LE, Yeakley W, et al: Recurrence of herpetic stromal keratitis after a conjunctival flap surgical
procedure. Am J Ophthalmol 114:231, 1992 102. Ficker LA, Kirkness CM, Rice NS, et al: The changing management and improved prognosis for corneal grafting in
herpes simplex keratitis. Ophthalmology 96:1587, 1989 103. Ficker LA, Kirkness CM, Rice NS, et al: Long-term prognosis for corneal grafting in herpes simplex keratitis. Eye 2(Part 4) :400, 1988 104. Larkin DFP: Corneal transplantation for herpes simplex keratitis. Br J Ophthalmol 82:107, 1998 105. Halberstadt M, Machens M, Gahlenbek KH, et al: The outcome of corneal grafting in patients with stromal keratitis of herpetic
and non-herpetic origin. Br J Ophthalmol 86:646, 2002 106. Cohen E, Laibson P, Arentsen J: Corneal transplantation for herpes simplex keratitis. Am J Ophthalmol 95:645, 1983 107. Foster CS, Duncan J: Penetrating keratoplasty for herpes simplex keratitis. Am J Ophthalmol 92:336, 1981 108. Langston R, Pavan-Langston D, Dohlman CH: Penetrating keratoplasty for herpetic keratitis. Trans Am Acad Ophthalmol Otolaryngol 79:577, 1975 109. Polack FM, Kaufman HE: Penetrating keratoplasty in herpetic keratitis. Am J Ophthalmol 73:908, 1972 110. Patten JT, Cavanagh HD, Pavan-Langston D: Penetrating keratoplasty in acute herpetic corneal perforations. Ann Ophthalmol 8:287, 1976 111. Pfister RR, Richards JS, Dohlman CH: Recurrence of herpetic keratitis in corneal grafts. Am J Ophthalmol 73:192, 1972 112. Pfister RR, Richards JS, Dohlman CH: Recurrence of herpetic keratitis in corneal grafts. Am J Ophthalmol 73:192, 1972 113. Beyer CF, Hill JM, Kaufman HE: Antivirals and interferons. Ophthalmol Clin North Am 2:51, 1989 114. Falcon MG: Rational acyclovir therapy in herpetic eye disease. Br J Ophthalmol 71:102, 1987 115. Green MT, Dunkel EC, Morris BL: Quantification of herpes simplex virus type 1 shed in pre-ocular
tear film of rabbits treated with acyclovir. Antimicrob Agents Chemother 20:580, 1981 116. Barney NP, Foster CS: A prospective randomized trial of oral acyclovir after penetrating keratoplasty
for herpes simplex keratitis. Cornea 13:232, 1994 117. Beyer CF, Arens MQ, Hill GA, et al: Oral acyclovir reduces the incidence of recurrent herpes simplex in rabbits
after penetrating keratoplasty. Arch Ophthalmol 107:1200, 1989 118. Van Rooij J, Rijneveld WJ, Remeijer L, et al: Effect of oral acyclovir after penetrating keratoplasty for herpetic keratitis. A
placebo controlled multicentered trial. Ophthalmology 110:1916, 2003 119. Herpetic Eye Disease Group: Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med 339:300, 1998 120. Lairson DR, Begley CE, Reynolds TF, et al: Prevention of herpes simplex virus eye disease: a cost effectiveness analysis. Arch Ophthalmol 122:108, 2003 121. Herpes Eye Disease Study Group: Predictors of recurrent herpes simplex keratitis. Cornea 20:123, 2001 |