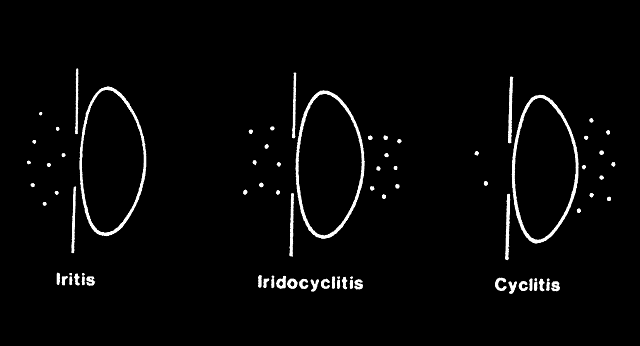
CILIARY INJECTION
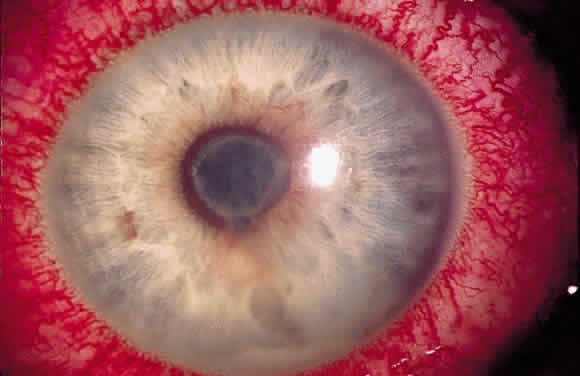
Ciliary injection, or “ciliary flush,” is manifest by a ring of dilated episcleral vessels radiating from the limbus. It should be distinguished from the deeper and more peripheral injection of scleritis and from the sectoral or diffuse injection of episcleritis. Overlying conjunctival injection may mask ciliary flush but topically applied neosynephrine blanches the overlying conjunctiva, allowing visualization of deeper episcleral vessels.
PUPILLARY MIOSIS OR IRREGULARITY
The pupil is typically small in patients with iritis because iris inflammation results in a release of prostaglandins, which constrict the pupil.4 One exception is patients with herpetic uveitis, who may present with a dilated pupil.5 The pupil in patients with anterior uveitis often becomes irregular and fixed because of the development of posterior synechiae.
BAND KERATOPATHY
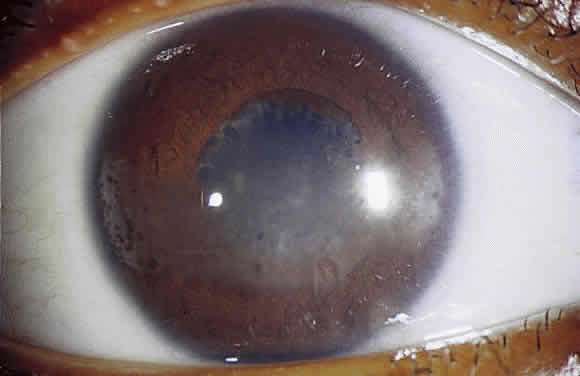
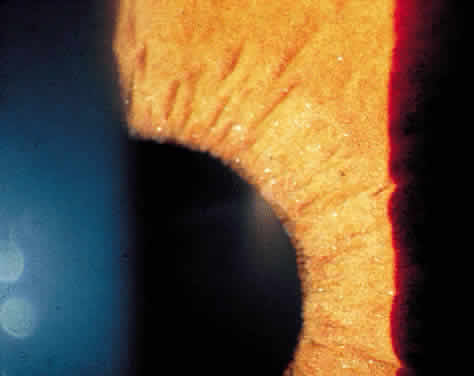
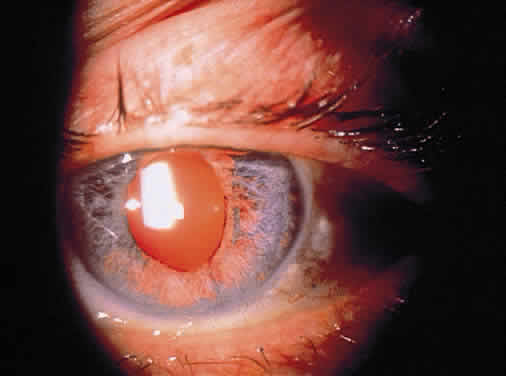
Long-standing chronic iridocyclitis, especially in children, may result in calcific band keratopathy, the deposition of calcium hydroxyapatite in the cornea at the level of Bowman's membrane. Band keratopathy usually begins as grayish-white opacities at the periphery of the interpalpebral region. The opacification may spread centrally and in time may form a complete band within the interpalpebral zone. A lucid interval is noted between the band and the limbus because Bowman's layer does not extend to the absolute limbus.6 Small clear areas are noted in the opacity, representing the location where corneal nerves penetrate Bowman's layer. These holes impart a “Swiss cheese” appearance to band keratopathy (Fig. 1). Band keratopathy should be distinguished from Vogt's limbal girdle and spheroidal degeneration. Occasionally, band keratopathy is atypical and starts centrally. Rarely, it forms a reticular pattern resembling lattice dystrophy called superficial reticular degeneration of Koby.7
KERATIC PRECIPITATES
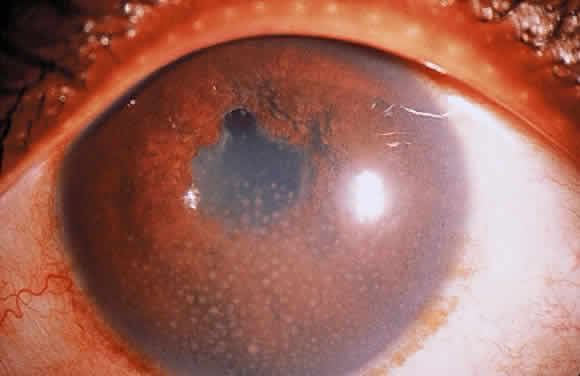
Clusters of inflammatory cells deposited on the endothelial surface of the cornea are known as keratic precipitates. The cells, which have been deposited from the aqueous humor, are often found inferiorly on the cornea in a linear vertical formation (Turk's line) or in the form of a base-down triangle (Arlt's triangle; Fig. 2). The inferior corneal distribution results from convection currents in the anterior chamber that rise along the warm iris and fall along the cool cornea. Exceptions to the rule of inferior distribution of keratic precipitates include inflammation secondary to herpes simplex and zoster, cytomegalovirus, and Fuchs' heterochromic iridocyclitis, which result in fine stellate keratic precipitates, often throughout the whole cornea. Sarcoidosis and VKH may also result in keratic precipitates distributed above the midline but these keratic precipitates tend to be larger and greasier in appearance than those associated with Fuchs' or herpetic iridocyclitis.
|
The cells that precipitate on the corneal endothelium reflect the composition of those in the aqueous humor. In acute inflammation, polymorphonuclear leukocytes predominate, whereas in chronic inflammation lymphocytes, plasma cells and pigment are more common.8 Fine fibrin dusting may also be present, alone or among the keratic precipitates. Fibrin is a prominent feature of the keratic precipitates of Fuchs' iridocyclitis, forming the bridges and stellate arms of the keratic precipitates.
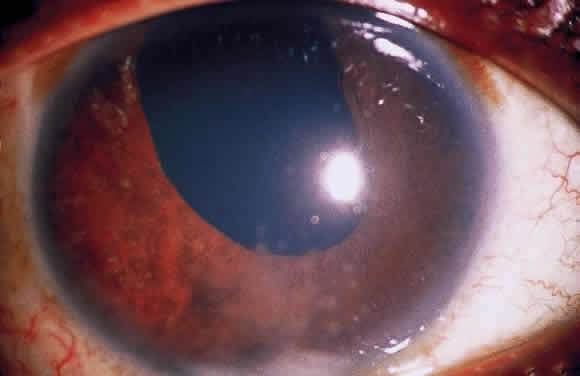
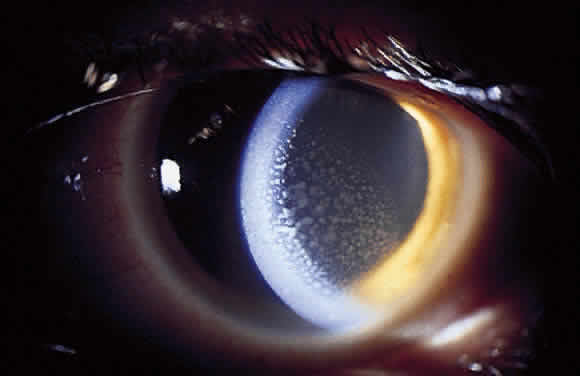
In addition to size, shape, and distribution of keratic precipitates, color may be an important characteristic. Fresh keratic precipitates tend to be white and round, whereas older keratic precipitates may be pigmented, faded, or have an irregular crenelated appearance. Descemet's membrane may cover old keratic precipitates, imparting a glassy or ghost-like appearance (Fig. 3). Large greasy-white keratic precipitates approaching 1 mm in diameter are often termed mutton-fat keratic precipitates and represent clusters of macrophages and epithelioid cells (Fig. 4). These are pathognomonic of granulomatous inflammation.
|
|
Although many keratic precipitates fade without sequelae, transient decompensation of the cornea may ensue. The endothelium may have the appearance of having guttae (cornea pseudoguttata) secondary to endothelial cell swelling.9 Permanent opacification of the inferior corneal endothelium has also been described in chronic iridocyclitis, resulting from fibrous metaplasia of the endothelium due to the presence of keratic precipitates10 (see Fig. 3). It is important to document size, color, distribution, and number of keratic precipitates at each visit. For example, one should describe 30 small white keratic precipitates and 5 medium pigmented keratic precipitates all located on the inferior cornea, rather than simply noting that the patient has keratic precipitates.
ANTERIOR CHAMBER FLARE
Normal aqueous humor is optically empty. If the slit-lamp beam is seen in the anterior chamber, it is termed “flare” and represents breakdown of the blood-aqueous barrier with exudation of protein. Flare is still graded according to the scheme proposed by Hogan in 195911 (Table 1). The light intensity of the slit lamp is turned to maximum, a short 1- to 2-mm slit is used, and the ray of light is directed at an oblique angle to the plane of the iris. Flare may also be measured using a laser flare meter or flare-cell meter, which quantifies anterior chamber protein by measuring light scattering of a helium-neon laser beam in the anterior chamber.12,13
TABLE 1. Grading of Anterior Chamber Flare
| Flare | Description |
| 0 | Complete absence |
| 1+ | Faint flare (barely detectable) |
| 2+ | Moderate flare (iris and lens details clear) |
| 3+ | Marked flare (iris and lens details hazy) |
| 4+ | 50 or more |
ANTERIOR CHAMBER CELLS
Cells in the aqueous humor usually result from active inflammation of the iris and ciliary body. Larger cells probably represent swollen macrophages or clumps of lymphocytes, whereas smaller cells may be individual lymphocytes. Studies of anterior chamber cells have shown that lymphocytes and monocytes predominate.14 T lymphocytes are the most common cell type overall,15 and polymorphonuclear leukocytes are common in hypopyon uveitis.14 Inflammatory anterior chamber cells are usually white and should be distinguished from pigmented cells, which may not indicate active inflammation. Pigmented cells may be uveal cells, melanin-containing macrophages, erythrocytes, or macrophages filled with pigment. Free pigment may also be found in the anterior chamber and should be distinguished from cells.
Cells in the anterior chamber are counted using the wide beam with a 1- to 2-mm long slit and graded according the method of Hogan and coworkers (Table 2).11 If the cells seem to be suspended rather than moving, there is probably extensive fibrin in the aqueous. Leukocytes may precipitate out of the anterior chamber to form a hypopyon (Fig. 5), and erythrocytes may layer out in a hyphema. It is important to examine the area of the inferior limbus, so that the presence of layered cells is not overlooked.
| Grade | Cells per Field |
| 0 | No cells |
| Rare | 1–2 |
| Occasional | 3–7 |
| 1+ | 7–10 |
| 2+ | 10–20 |
| 3+ | 20–50 |
| 4+ | 50 or more |
|
CELLS IN THE ANTERIOR VITREOUS
The density of cells in the anterior vitreous (retrolental space) should be estimated after pupillary dilation and compared with the density of the cells in the anterior chamber. A diagram indicating the density of the cells in these two locations is a useful method of documentation (Fig. 6).
IRIS ABNORMALITIES
Some iris nodules are analogous to keratic precipitates in representing cells that have precipitated out of the aqueous humor. Other nodules may represent inflammatory foci on or within the iris.
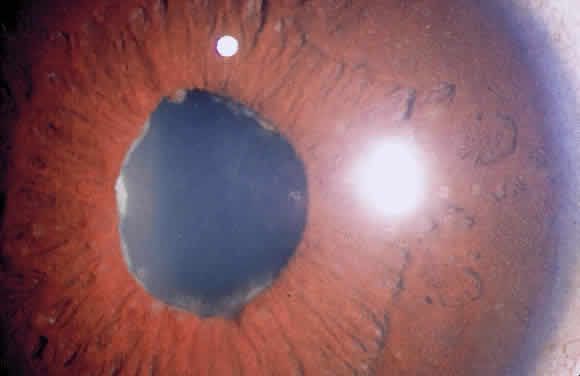
Iris nodules may be gray or white and translucent and are often covered with pigment. Masses at the pupillary border are called Koeppe nodules, whereas those on the anterior surface of the iris are called Busacca nodules (Fig. 7; see Fig 2). Busacca nodules are evidence of granulomatous inflammation, and Koeppe nodules are often located in areas that subsequently develop posterior synechiae. The presence and location of nodules should be carefully diagrammed, and every effort must be made to keep the pupil moving, thereby preventing the development of permanent iridolenticular adhesions.
|
Posterior synechiae may also develop without nodule formation because fibrin in the aqueous can induce adhesions to the lens. Later, fibrous organization may make adhesions pharmacologically unbreakable, and the formation of synechiae during treatment indicates inadequate therapy. If posterior synechia formation proceeds unchecked, pupillary block glaucoma may develop (Fig. 8). Peripheral anterior synechiae (PAS) in the anterior chamber angle may result from chronic shallowing of the anterior chamber because of posterior synechiae and pupillary block. They may also result from fibrous organization of inflammatory exudates and precipitates in the angle. Rubeosis may also occur in severe long-standing uveitis and lead to PAS. Progression of PAS may lead to angleclosure glaucoma.
|
In cases of chronic iritis, tiny refractile crystals may be seen on the surface of the iris, probably representing Russell bodies16,17 (Fig. 9). The iris of patients with chronic iritis may demonstrate prominent iris vessels that follow the normal anatomy. These congested normal vessels must be distinguished from neovascular rubeotic vessels, which tend to arborize at random. Clinically, it may sometimes be difficult differentiate the two, and both types of vessels leak on fluorescein angiography.18
|
The iris often becomes atrophic in patients with chronic iridocyclitis. Atrophy of the posterior pigmented epithelium of the iris is more common in herpetic uveitis and may result in iris transillumination defects (Fig. 10). Anterior stromal atrophy is more characteristic of Fuchs' iridocyclitis and often results in heterochromia. The irides should therefore be examined by external inspection with the room lights on to document any heterochromia and at the slit lamp to document transillumination defects.
|
INTRAOCULAR PRESSURE
Intraocular pressure (IOP) must be monitored initially and at each subsequent examination of every uveitis patient. Patients with acute anterior uveitis usually present with low IOP, secondary to infiltration of the ciliary body by inflammatory cells and subsequent reduction of aqueous production. The release of prostaglandins may also play a role in decreasing IOP.19 In contrast, patients with severe inflammation may have elevated IOP because of inflammation in the area of the trabecular meshwork or blockage of the angle by cells and debris.20 Exceptions to the rule that most patients with acute iridocyclitis have low IOP are seen with herpes simplex uveitis, herpes zoster uveitis, PosnerSchlossman syndrome, and toxoplasmosis. These patients often have elevated IOP early in the course of their disease.21–25
Patients with chronic iridocyclitis frequently develop elevated IOP. This elevation of IOP may result from PAS, trabecular scarring, pupillary block resulting from posterior synechiae, rubeosis irides, or corticosteroid therapy. Patients may develop elevated IOP after any form of corticosteroids, including topical drops; subtenon or subconjunctival injection; and intravenous, oral, and inhaled formulations. Any patient receiving local or systemic corticosteroids must have IOP monitored at each visit. Some patients do not initially respond to steroids with an elevation in IOP but become steroidresponders after months or even years of therapy.26 Some of the delayed steroid response may be due to progressive trabecular compromise resulting from inflammation and from increased aqueous production because of ciliary body recovery.
LENS OPACITIES
Chronic or recurrent acute iridocyclitis may lead to posterior subcapsular cataract formation. Lysophosphatidyl choline, which is elevated in the anterior chamber during inflammation, may contribute to cataract formation.27–29 Steroids also contribute to the development of cataract in uveitis patients. They are well-known cataractogenic agents, although there does not appear to be a strong relation between the dose of corticosteroids and the onset of cataract30; that is, some people develop cataracts after a relatively short course of steroids, whereas others seem fairly resistant. Thus, it is almost impossible to tell whether the treatment or the disease is the major contributing factor for cataract formation in a given uveitis patient.
Fibrinous membranes may develop over the surface of the lens in some cases of iridocyclitis. The underlying lens may be clear but the epilenticular membrane prevents a clear view of the lens and decreases vision. This is particularly common in patients with juvenile rheumatoid arthritis-related iridocyclitis, and the membrane may occasionally be peeled off the lens without the need for cataract surgery.
VITREOUS OPACITIES AND HAZE
Inflammatory posterior vitreous opacities have historically been graded by direct ophthalmoscopy.31 The method most commonly used is that described by Nussenblatt and associates,32 which uses the indirect ophthalmoscope (Table 3).
TABLE 3. Classification of Vitreous Haze
| Grade | Description |
| 0 | No haze |
| Trace | Slight blurring of optic disc margin |
| 1+ | Slightly blurred optic nerve and vessels |
| 2+ | Moderately blurred optic nerve and vessels |
| 3+ | Optic nerve head border blurry but visible |
| 4+ | Optic nerve head obscured |
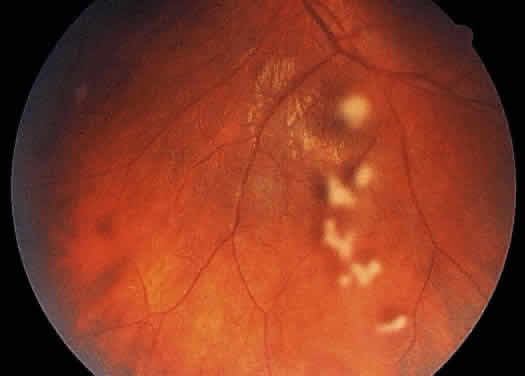
Posterior vitreous opacities are best studied with contact or noncontact (78 diopter, 90 diopter, or superfield lens) slit-lamp biomicroscopy. Exudates or clumps of inflammatory cells may be seen anywhere in the vitreous. They may line the posterior surface of a posterior vitreous detachment, forming so-called vitreous precipitates, which are analogous to keratic precipitates and are common in toxoplasmosis. They may also be seen within the vitreous or on the retinal surface. Large clumps of vitreous cells are often termed “snowballs” (Fig. 11). Traction bands may form in the vitreous and result in the development of vitreous hemorrhage or retinal detachment.
|
CILIARY BODY ABNORMALITIES
The pars plana of the ciliary body may be seen by indirect ophthalmoscopy with scleral depression. This is particularly important in cases of suspected pars planitis, where one may see “snowbanking” of inflammatory cells and neovascular membranes. The pars plicata of the ciliary body is more difficult to see, and the ultrasound biomicroscope may be used to provide information about its position and morphology.33–35 It may also be used to provide information about angle structures, ciliary body, and peripheral retina, particularly in patients without clear media.
Cyclitic membranes that form between the ciliary processes may lead to ciliary body detachment, hypotony, and phthisis bulbi. This process most frequently occurs in long-standing cases of iridocyclitis. Extension of the cyclitic membranes posteriorly may lead to tractional retinal detachment. These membranes may be difficult to detect. Sometimes they are seen in the retrolental space at the slit lamp but in other cases they are not seen clinically. Their presence may often be inferred because of profound hypotony that is unresponsive to anti-inflammatory therapy, and ultrasound biomicroscopy may be confirmatory.
FUNDUS ABNORMALITIES
Every patient with uveitis requires a complete fundus examination. Posterior foci inflammation or infection may be missed if one examines the anterior chamber only. For example, patients with cytomegalovirus retinitis or toxoplasmic retinochoroiditis may present with anterior chamber inflammation. If a complete dilated fundus examination is not performed, the patient may be misdiagnosed as having noninfectious iritis or iridocyclitis. Indirect ophthalmoscopy is necessary to see the peripheral retina. The macula should also be examined with slit-lamp biomicroscopy in all patients with uveitis because cystoid macular edema is a frequent cause of decreased vision in patients with anterior, intermediate, and posterior inflammation. It should be suspected in cases where visual acuity is decreased, even when the macula does not appear grossly edematous. Fluorescein angiography is a helpful adjunct to clinical examination, especially in cases with a compromised view of the fovea. Other causes of decreased visual acuity in patients with uveitis include epiretinal membranes and subretinal neovascularization. It is important not to overlook these findings because laser or surgical therapy may improve prognosis.
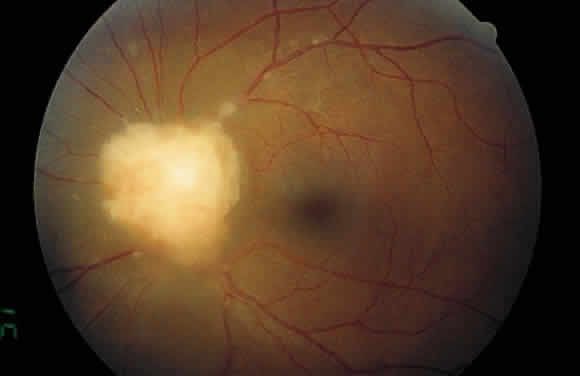
Examination of the posterior segment of the eye must also include an examination of the optic nerve head. Disc edema or hyperemia is frequent in uveitis patients, often preceding the development of macular edema. Neovascularization of the disc is another abnormality that may develop in patients with severe uveitis.36 The optic nerve head may also be a site of granuloma formation (Fig. 12).
|
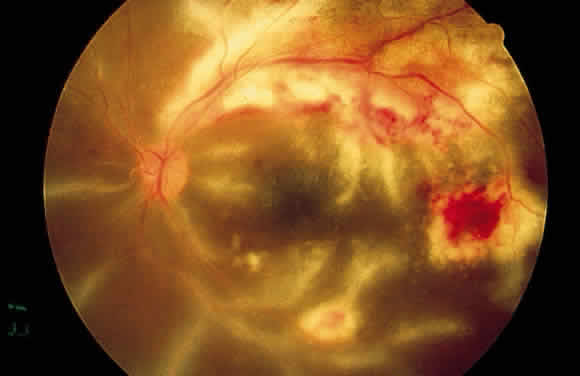
The retinal vessels should be examined for evidence of vasculitis, noting whether the vasculitis affects primarily the veins (phlebitis) or the arteries (arteritis). Patients with sarcoid uveitis commonly have extensive periphlebitis, whereas patients with Behçet's disease may have more of an arteritic picture. Patients with herpetic retinitis may have extensive arteritis and phlebitis, which produce the appearance of “frosted branch angiitis”37 (Fig. 13).
Examination of the fundus also includes an evaluation of the choroid because choroidal lesions are seen in many infectious and noninfectious uveitides. They may range in size from small Dalen-Fuchs—like nodules to large patches of choroidal infiltration, as seen in large-cell lymphoma. Choroidal granulomas may resolve, leaving “punched-out” atrophic lesions, such as the peripheral spots seen in the ocular histoplasmosis syndrome.
A good history and clinical examination are invaluable in patients with uveitis. They are necessary for the development of a differential diagnosis and cannot be replaced by a barrage of nondirected laboratory tests. Once a good history has been elicited—including ocular and extraocular symptoms—and a detailed clinical examination performed, a rational differential diagnosis can be formulated and a directed work-up obtained.